Abstract
Background
The mixed epithelial stromal tumour is morphologically characterised by a mixture of solid and cystic areas consisting of a biphasic proliferation of glands admixed with solid areas of spindle cells with variable cellularity and growth patterns. In previous reports the seminal vesicle cystoadenoma was either considered a synonym of or misdiagnosed as mixed epithelial stromal tumour. The recent World Health Organisation Classification of Tumours considered the two lesions as two distinct neoplasms. This work is aimed to present the low-grade epithelial stromal tumour case and the review of the literature to the extent of establishing the true frequency of the neoplasm.
Case presentation
We describe a low-grade epithelial stromal tumour of the seminal vesicle in a 50-year-old man. Computed tomography showed a 9 × 4.5 cm pelvic mass in the side of the seminal vesicle displacing the prostate and the urinary bladder. Magnetic resonance was able to define tissue planes between the lesion and the adjacent structures and provided useful information for an accurate conservative laparotomic surgical approach. The histology revealed biphasic proliferation of benign glands admixed with stromal cellularity, with focal atypia. After 26 months after the excision the patient is still alive with no evidence of disease.
Conclusion
Cystoadenoma and mixed epithelial stromal tumour of seminal vesicle are two distinct pathological entities with different histological features and clinical outcome. Due to the unavailability of accurate prognostic parameters, the prediction of the potential biological evolution of mixed epithelial stromal tumour is still difficult. In our case magnetic resonance imaging was able to avoid an exploratory laparotomy and to establish an accurate conservative surgical treatment of the tumour.
Similar content being viewed by others
Background
The mixed epithelial and stromal tumour (MEST) is morphologically characterised by a mixture of solid and cystic areas with a biphasic proliferation of glands admixed with solid areas of spindle cells, with variable cellularity and growth patterns. We report a low grade MEST of the seminal vesicle and we emphasize the diagnostic criteria and the different diagnosis as cystoadenoma.
Case presentation
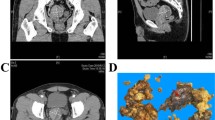
A 50-year-old man was admitted in November 2005 to our hospital with disuria, fever and gradual decrease in urinary stream for several months. On ultrasound (US) a hynomogeneous, hypoechoic pelvic mass of the posterior side of the bladder was demonstrated. Abdominal computed tomography (CT) revealed a 9 × 4.5 cm pelvic mass in the side of the seminal vesicles displacing the prostate and urinary bladder. On precontrast CT, the mass showed fluid heterogeneous content, well-defined margins, and irregular thin enhanced internal septa after intravenous contrast material administration. On axial spin-echo T1-weighted and axial and sagittal T2-weighted (Fig. 1a–c) magnetic resonance imaging (MRI), a large well defined multilocular pelvic mass was revealed in the side of the seminal vesicles, contiguous to the posterior wall of the urinary bladder, above the prostate and anterior to the rectum. On T1 and T2-weighted images, the mass showed internal septa that delimited heterogeneous iso- hyperintense areas with proteinaceus content. On axial T1-weighted fat-suppression gradient-echo images, before and after intravenous administration of gadopentetate-dimeglumine (gadolinium-DTPA), no enhancement of the mass occurred. The exploratory laparotomy revealed a retrovesical mass, which was well defined and not firmly adherent anteriorly to the bladder. It was supposed that the origin of the mass might be the right seminal vesicle. The tumour was totally dissected from the attachments to the bladder anteriorly ad rectum posteriorly, which required the removal of the left seminal vesicle and a portion of both vasa deferens. 14 months after surgery, the patient is currently alive with no evidence of disease. The tumour measured 9 × 6 × 6 cm and consisted of an oval rubbery mass. The cut surfaces showed multilocular cysts of variable sizes and shapes filled with gelatinous material (Fig. 2). The histological examination revealed two distinctive but intermixed components, one glandular and the other stromal (Fig. 3). The glandular proliferation was characterized by cystically dilated glandular spaces, containing pale eosinophilic intraluminal secretions and lined by one to two layers of cuboidal or low columnar cells. There was either no significant cytologic atypia or appreciable mitotic activity. The epithelial cells were uniformly reactive against all keratin proteins (AE1/AE3, CAM5.2, and high-molecular-weight keratin). Monoclonal and polyclonal carcinoembryonic antigen (CEA), prostate acid phosphatase (PAP) and prostate – specific antigen (PSA) stains gave negative results. The stromal component was composed by extensive loosely cellular areas with mixoid content. The stromal cells were spindle-shaped and showed pleomorphism. The stroma was at least focally densely cellular and tended to condense around distorted glands. No mitoses were found (Fig. 4). The spindle-shaped cells showed strong reactivity for vimentin, CD34, and patchy weaker reactivity (30%) for α-smooth muscle actin (Fig. 5a–c) and desmin, but were negative for cytokeratin and PSA. We preferred a diagnosis of low grade MEST because of the presence of cellular pleomorphism of the stromal component. 26 months after surgery, the patient is still alive, with no evidence of disease.
Axial spin-echo T1-weighted (a) and axial (b) and sagittal T2-weighted (c) MRI showed a large, well-defined, multilocular pelvic mass in the side of the seminal vesicles, contiguous to the posterior wall of the urinary bladder and the prostate. On T1 and T2-weighted images the mass showed internal septations that delimited heterogeneous iso- hyperintense areas with proteinaceus content.
Discussion
In our case the low-grade MEST diagnosis was made in accordance with the World Health Organization (WHO) classification criteria [1]. The lesion arose from the seminal vesicle and did not: a) present normal tissue, b) invade the prostate, and it was not immunoreactive for monoclonal and polyclonal CEA, PAP and PSA. The glandular proliferation was benign. Mitosis and atypia of the epithelium were not found. The stromal cellularity was mostly pronounced in the tissue adjacent to intratumoural glands. Focal cellular pleomorphism was present, but mitoses were not found. The examined lesion was categorized into low-grade MEST because of the presence of atypia in the spindle-shaped cells. The former literature reviews reported MEST under different names: cystomyoma [2], cystadenoma [3–12], mesonephric hamartoma [13], papillary cystadenoma [14], cystic epithelial-stromal tumour [15], mullerian adenosarcoma-like tumour [16], cystosarcoma phyllodes [17, 18], phyllodes tumour [19], epithelial stromal tumour with phyllodes tumour-like features [20], benign fibroepithelial tumour [21].
These tumours have been considered to represent morphological variants of the same neoplasm, which may reveal local recurrence but not malignant transformation [7, 10]. In the WHO classification [1], the seminal vesicle cystoadenoma (SVC) was excluded from the category of MESTs and considered as a distinct neoplasm; it should be histologically distinguished from MEST by its non-neoplastic stroma. Consequently the previous reports of MESTs have been critically revised (table 1). In the reviews of 10 and 14 MESTs, Baschinsky et al., [9] and Son et al., [19] reported respectively 6 and 8 cases of SVC. According to WHO criteria, the cases of SVC reported by Lagalla et al., [8], Bullock et al [6], Damjanov and Apic [22], Lundhus et al., [23], should be excluded from MESTs. This is because the description of stromal component is absent [6] or because microscopically they showed a non-neoplastic stromal component, that is typical of benign glandular tumours. The SVC described by Soule and Dockerty [3], Baschinky et al., [9], Mazzucchelli et al., [7], Gil et al., [11], the mesonephric hamartoma of Kinas et al., [13], the benign fibroepithelial of Zanetti et al., [20], should be considered MEST, because all these tumours showed simultaneous ductal and stromal proliferations. In the histological grading of stromal component, the WHO classification categorised MESTs in low or high grade, depending on mitotic activity and necrosis [1]. But the number of mitosis is not specified. In the high-grade MEST the stroma should be at least focally densely cellular and condensed around distorted glands. In the differential diagnosis between low and high-grade MEST the histological features reported by WHO classification are not exhaustive to achieve a conclusive diagnosis. The WHO rejected the concept of phylloides tumour despite the evidence that it is a separate entity from cystadenoma. In the MEST review of Son et al., [19] and Hoshi et al., [20] the classification of Baschinsky et al., [9] in low, intermediate, high-grade MEST was reported. Surprisingly this distinction is not described in the original paper of Baschinsky et al., [11]. In the description of cystosarcoma phyllodes, Fain et al., [17] examined the tumours reported by Mazur et al., [15] and Soule and Dockerty [3]: obviously the spectrum of phyllodes tumour reported by Fain et al., may be not accepted as conclusive because only three cases were considered. In our case US was only useful to detect the lesion, but both its site and relations with adjacent organs were not established. CT confirmed the presence, size, location, internal consistency, extension of the primary tumour, and the absence of distant metastases. MRI was useful in establishing the tumour origin from the seminal vesicles and its relations with the adjacent organs (Fig. 1). MRI with fat-suppression and administration of paramagnetic contrast agent is recommended to demonstrate the absence of tumour vascularisation and to indicate the benign non-vascular nature of the mass.
Conclusion
SVC and MEST are two distinct pathological entities with different histological features and clinical outcome. Predicting the potential biological behaviour of MEST remains difficult because accurate prognostic parameters are not available. In our case MRI was able to avoid an exploratory laparotomy and to establish an accurate conservative surgical treatment of the tumour.
Consent
Written informed consent was obtained from the patient for publication of this case report.
Abbreviations
- MEST:
-
mixed epithelial and stromal tumour
- US:
-
ultrasound
- WHO:
-
World Health Organization
- CT:
-
Computed tomography
- MRI:
-
magnetic resonance imaging
- CEA:
-
carcinoembryonic antigen
- PAP:
-
prostate acid phosphatase
- PSA:
-
prostate specific antigen.
References
Eble JN, Sauter G, Epstein JI, Sesterhenn IA, (eds): Pathology and genetics of tumours of the urinary system and male genital organs. World Health Organisation Classification of Tumours. 2004, IARC Press, Lyon, 214-215.
Plaut A, Standard S: Cystomyoma of seminal vesicle. Ann Surg. 1944, 119: 253-261. 10.1097/00000658-194402000-00009.
Soule EH, Dockerty MB: Cystadenoma of the seminal vesicle, a pathologic curiosity. Report of a case and review of the literature concerning benign tumours of the seminal vesicle. Staff Meet Mayo Clin. 1951, 26: 406-16.
Damjanov I, Apic R: Cystadenoma of seminal vesicles. J Urol. 1974, 111: 808-809.
Lundhus E, Bundgaard N, Sorensen FB: Cystadenoma of the seminal vesicle. Scand J Urol Nephrol. 1984, 18: 341-342.
Bullock KN: Cystadenoma of the seminal vesicle. J R Soc Med. 1988, 81: 294-295.
Mazzucchelli L, Studer UE, Zimmermann A: Cystadenoma of the seminal vesicle: case report and literature review. J Urol. 1992, 147: 1621-1624.
Lagalla R, Zappasodi F, Lo Casto A, Zenico T: Cystadenoma of the seminal vesicle: US and CT findings. Abdom Imaging. 1993, 18: 298-300. 10.1007/BF00198130.
Baschinsky DY, Niemann TH, Maximo CB, Bahnson RR: Seminal vesicle cystadenoma: a case report and literature review. Urology. 1998, 51: 840-845. 10.1016/S0090-4295(97)00711-5.
Santos LD, Wong CS, Killingsworth M: Cystadenoma of the seminal vesicle: report of a case with ultrastructural findings. Pathology. 2001, 33: 399-402. 10.1080/00313020120063072.
Gil AO, Yamakami LY, Genzini T: Cystadenoma of the seminal vesicle. Int Braz J Urol. 2003, 29: 434-436. 10.1590/S1677-55382003000500009.
Lee CB, Choi HJ, Cho DH, Ha US: Cystadenoma of the seminal vesicle. Int J Urol. 2006, 13: 1138-1140.
Kinas H, Kuhn MJ: Mesonephric hamartoma of the seminal vesicle: a rare cause of a retrovesical mass. N Y State J Med. 1987, 87: 48-49.
Raghuveer CV, Nagarajan S, Aurora AL: Papillary cystadenoma of seminal vesicle: a case report. Indian J Pathol Microbiol. 1989, 32: 314-315.
Mazur MT, Myers JL, Maddox WA: Cystic epithelial-stromal tumour of the seminal vesicle. Am J Surg Pathol. 1987, 11: 210-217. 10.1097/00000478-198703000-00006.
Laurila P, Leivo I, Mäkisalo H, Ruutu M, Miettinen M: Müllerian adenosarcomalike tumour of the seminal vesicle. A case report with immunohistochemical and ultrastructural observations. Arch Pathol Lab Med. 1992, 116: 1072-1076.
Fain JS, Cosnow I, King BF, Zincke H, Bostwick DG: Cystosarcoma phyllodes of the seminal vesicle. Cancer. 1993, 71: 2055-2061. 10.1002/1097-0142(19930315)71:6<2055::AID-CNCR2820710621>3.0.CO;2-W.
Abe H, Nishimura T, Miura T: Cystosarcoma phyllodes of the seminal vesicle. Int J Urol. 2002, 9: 599-601. 10.1046/j.1442-2042.2002.00515.x.
Son HJ, Jeong YJ, Kim JH, Chung MJ: Phyllodes tumour of the seminal vesicle: case report and literature review. Pathol Int. 2004, 54: 924-929. 10.1111/j.1440-1827.2004.01779.x.
Hoshi A, Nakamura E, Higashi S, Segawa T, Ito N, Yamamoto S, Kamoto T, Ogawa O: Epithelial stromal tumour of the seminal vesicle. Int J Urol. 2006, 13: 640-642. 10.1111/j.1442-2042.2006.01380.x.
Zanetti GR, Gazzano G, Trinchieri A, Magri V, Bosari S, Montanari E: A rare case of benign fibroepithelial tumour of the seminal vesicle. Arch Ital Urol Androl. 2003, 75: 164-165.
Damjanov I, Apiæ R: Cystadenoma of seminal vesicles. J Urol. 1974, 111: 808-809.
Lundhus E, Bundgaard N, Sørensen FB: Cystadenoma of the seminal vesicle. A case report. Scand J Urol Nephrol. 1984, 18: 341-342.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
IF collected the tissue specimen, made the histological diagnosis and images, revised the manuscript. IP made the radiological diagnosis, prepared radiological images. BM conceived the study and revised the manuscript. ML, FF, GP: collected clinical records and wrote the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Monica, B., Larosa, M., Facchini, F. et al. Low grade epithelial stromal tumour of the seminal vesicle. World J Surg Onc 6, 101 (2008). https://doi.org/10.1186/1477-7819-6-101
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7819-6-101