Abstract
Background
Staphylococcus aureus and S. epidermidis biofilms differ in structure, growth and regulation, and thus the high-throughput method of evaluating biofilm susceptibility that has been published for S. epidermidis cannot be applied to S. aureus without first evaluating the assay's reproducibility and reliability with S. aureus biofilms.
Methods
Staphylococcus aureus biofilms were treated with eleven approved antibiotics, lysostaphin, or Conflikt®, exposed to the oxidation reduction indicator Alamar blue, and reduction relative to untreated controls was determined visually and spectrophotometrically. The minimum biofilm inhibitory concentration (MBIC) was defined as ≤ 50% Alamar blue reduction and a purple/blue well 60 min after the addition of Alamar blue. Because all of the approved antibiotics had MBICs >128 μg/ml (most >2048 μg/ml), lysostaphin and Conflikt®, with relatively low MBICs, were used to correlate Alamar blue reduction with 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction and viable counts (CFU/ml) for S. aureus ATCC 29213 and three clinical isolates. Alamar blue's stability and lack of toxicity allowed CFU/ml to be determined from the same wells as Alamar blue absorbances.
Results
Overall, Alamar blue reduction had excellent correlation with XTT reduction and with CFU/ml. For ATCC 29213 and two clinical isolates treated with lysostaphin or Conflikt®, Alamar blue reduction had excellent correlation with XTT reduction (r = 0.93-0.99) and with CFU/ml (r = 0.92-0.98). For one of the clinical isolates, the results were moderately correlated for Conflikt® (r = 0.76, Alamar blue vs. XTT; r = 0.81, Alamar blue vs. CFU/ml) and had excellent correlation for lysostaphin (r = 0.95, Alamar blue vs. XTT; r = 0.97, Alamar blue vs. CFU/ml).
Conclusion
A reliable, reproducible method for evaluating biofilm susceptibility was successfully applied to S. aureus biofilms. The described method provides researchers with a simple, nontoxic, relatively inexpensive, high throughput measure of viability after drug treatment. A standardized biofilm Alamar blue assay should greatly increase the rate of discovery of S. aureus biofilm specific agents.
Similar content being viewed by others
Background
In the U.S., one million nosocomial infections each year are related to infections caused by biofilms on implanted devices [1]. Mortality for septicemias associated with vascular devices ranges from 20-40% [2], and intravenous catheters are the most common cause of nosocomial septicemia [3]. Staphylococcus aureus and S. epidermidis are the most common infectious agents associated with foreign device infections [4–6], and are found in biofilms in a wide range of other diseases, including endocarditis and osteomyelitis [7].
In vitro surface-associated and in vivo device-associated bacterial biofilms are generally quite resistant to antibiotic treatment [7, 8]. Despite decades of research in this area, treatment options are limited. A factor contributing to this unmet need is the lack of a standardized method for determining the drug susceptibility of bacterial biofilms. Several methods are available, but are limited by long processing times, incompatibility with high throughput, expensive reagents or equipment, or the method measures mass instead of viability [9–13]. A simple high-throughput assay that measures viability has been standardized for S. epidermidis [14] and Candida albicans [15]. This colorimetric Alamar blue (AB) assay was reliable, reproducible and had good to excellent correlation with two other biofilm susceptibility methods, 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction and viable counts (CFU/ml) for four strains of S. epidermidis.
Alamar blue has been used extensively in mammalian cell culture cytotoxicity assays and planktonic bacterial and fungal susceptibility assays, and is a simple, one-step procedure, in which metabolic activity results in the chemical reduction of AB. Alamar blue is reduced by FMNH2, FADH2, NAHD, NADPH and the cytochromes (product literature, Trek Diagnostic Systems). Alamar blue both fluoresces and changes color in response to chemical reduction, and the extent of the conversion is a reflection of cell viability (product literature, Trek Diagnostic Systems). Continued growth maintains a reduced environment while inhibition of growth maintains an oxidized environment. Alamar blue is water soluble, so the washing/fixation/extraction steps required in other commonly used cell proliferation assays are not required. Data may be collected with the naked eye, or for increased sensitivity, with either fluorescence-based or absorbance-based instruments. Alamar blue is also nontoxic to both the investigator and to the cells of interest, so it is safe to work with, easily disposed of and less likely to interfere with normal metabolism in test cells. In addition, AB is stable, so long incubations are possible, as are kinetic studies.
There are numerous differences in biofilm structure, growth and regulation in S. aureus and S. epidermidis [16–18]. Biofilms from different species of Candida also differ in structure and growth [19, 20], so the colorimetric XTT susceptibility assay must be standardized for each species [21]. The high-throughput method of evaluating biofilm susceptibility that has been published for S. epidermidis cannot be applied to S. aureus without first evaluating the assay's reproducibility and reliability with S. aureus biofilms.
Methods
Strains
Staphylococcus aureus ATCC 29213 (wound isolate) and three clinical isolates (520009, 520016, 520020; Arizona Department of Health Services, Phoenix, AZ) were maintained on Mueller-Hinton agar (MHA) at 35°C. To confirm biofilm formation, S. aureus ATCC 29213, a known biofilm former [10], and the three clinical isolates were grown on glass and polystyrene for 24 and 48 h at 35°C without shaking, stained with Congo red, rinsed, drained and adhesion relative to 17 other clinical isolates was ranked.
Antimicrobial agents
Clindamycin, bacitracin, vancomycin, ciprofloxacin, gentamicin, rifampin, nitrofurazone and enrofloxacin were obtained from ICN. Ceftriaxone, penicillin and oxacillin were from Sigma. Clindamycin, rifampin, nitrofurazone and enrofloxacin were dissolved in sterile dimethyl sulfoxide (DMSO). The remaining antibiotics and lysostaphin were dissolved in sterile H2O. Conflikt® (Decon Laboratories Inc.) was used as received.
Alamar blue biofilm susceptibility assay
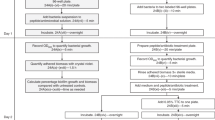
The AB biofilm susceptibility assay and calculation of the percent reduction of AB were performed as described for S. epidermidis [14]. Alamar blue (Trek Diagnostic Systems), which is blue-colored in its oxidized state, is reduced in metabolically active cells to the pink colored resorufin. Alamar blue was aliquoted and stored at -80°C. Prior to each experiment, AB was brought to room temperature and vortexed. Exposure of AB to light was minimized throughout experiments. Isolated colonies from 18-22 MHA plates were used to prepare inocula. Assays were performed in flat bottom, polystyrene, non-tissue culture treated microtiter plates containing 5 × 105 CFU/ml in MHIIB media, with final well volumes of 100 μl. Plates were incubated at 37°C without shaking. Two fold-dilutions of drugs in cation-adjusted MHIIB were prepared external to the plates. After 24 h, 50 μl was removed from all experimental and control wells, and 50 μl of the appropriate drug dilution added. Biofilms were exposed to drugs for 20 h at 37°C without shaking. After 20 h, 5 μl AB was added to wells (105 μl total volume), the plates shaken gently and incubated for 1 h at 37°C. Plates were gently shaken again and absorbance at 570 nm and 600 nm obtained in a Perkin Elmer Wallac Victor3 microplate reader. For experiments with multiple time points, plates were kept in a 37°C incubator between absorbance readings. Controls included media alone, media plus AB, media plus AB plus drug dilution, and cells plus media plus AB. Percent reduction of AB was calculated using the manufacturer's formula, with replacement of their negative control, which contains only media plus AB, with a more robust negative control, media plus AB plus a drug concentration equal to each experimental well:

where,
εox = molar extinction coefficient of Alamar blue oxidized form (blue)
εred = molar extinction coefficient of Alamar blue reduced form (pink)
A = absorbance of test wells
A' = absorbance of negative control well
λ1 = 570 nm
λ2 = 600 nm
The AB minimum biofilm inhibitory concentration (MBIC) was defined as the lowest drug concentration resulting in ≤ 50% reduction of AB and a purple/blue well 60 min after the addition of AB [14]. Assays were performed at least twice, and the average % reduction used to determine the AB MBIC.
Alamar blue planktonic susceptibility assay
Planktonic susceptibility testing of S. aureus was performed by the CLSI reference broth microdilution assay (BMA) [22] as previously described [14]. Assays were performed at least twice, and the average % reduction used to determine the MIC. The AB MIC was defined as the lowest drug concentration resulting in ≤ 50% reduction of AB and a purple/blue well 60 min after the addition of AB [14].
Biofilm 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay
The biofilm XTT reduction assay and calculation of percent formazan production were performed exactly as described for S. epidermidis [14].
Biofilm CFU/ml assay
CFU/ml were obtained from the same wells used to obtain biofilm AB absorbances as previously described [14].
Correlation of Alamar blue reduction to XTT reduction and CFU/ml
For correlation experiments, AB, XTT and CFU/ml assays were performed the same day using a single inoculum. Experiments were repeated at least twice. Pearson's two-tailed correlations were calculated with Prism 4 software using averaged data from the entire range of drug concentrations.
Results
When the relative adhesion of 20 S. aureus clinical isolates and biofilm positive ATCC 29213 was scored, S. aureus ATCC 29213 and clinical isolates 520009, 520016 and 520020 ranked medium to high on both glass and polystyrene (three isolates were biofilm-negative on both substrates; the remaining isolates ranked medium to high on one or both substrates).
In order to make the assay as efficient as possible, the shortest possible AB reduction endpoint was determined as for S. epidermidis [14]. Twenty-four hour S. aureus biofilms were treated for 20 h with ciprofloxacin, lysostaphin or Conflikt®. Alamar blue was then added and absorbance determined 30, 60 and 90 min after the addition of AB. The susceptibility pattern was clear at 60 min (data not shown), just as with S. epidermidis [14], so this time point was chosen as the endpoint for absorbance readings in all experiments.
Prior to determining biofilm susceptibility, MICs for a variety of antibiotics with different mechanisms of action were determined for planktonic-grown S. aureus ATCC 29213 (Table 1). Turbidometric and AB MICs for planktonic grown strains were identical or within one twofold dilution (data not shown). MICs and MBICs were defined as the lowest drug concentration resulting in ≤ 50% reduction of AB, and in the case of noncolored compounds, a purple/blue well 60 min after the addition of AB (for colored compounds, AB reduction can only be determined spectrophotometrically). Alamar blue MBICs increased at least five-fold relative to planktonic AB MICs (Table 1), consistent with previous reports of many-fold increases in drug resistance of biofilm vs. planktonic-grown strains [7]. Even at doses of 2048 μg/ml, S. aureus ATCC 29213 biofilms were resistant to all antibiotics tested (Table 1). Nitrofurazone and ciprofloxacin could not be tested at doses higher than indicated (Table 1) because they precipitate at 512 μg/ml and 256 μg/ml, respectively.
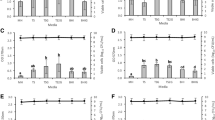
As no antibiotics inhibited S. aureus ATCC 29213 biofilms, we investigated an enzyme with reported activity against S. aureus biofilms, and a widely used disinfectant. Lysostaphin is an endopeptidase that cleaves the cross-linking pentaglycine bridges of the cell wall of staphylococci [23]. At low concentrations, lysostaphin kills S. aureus biofilms and, in addition, disrupts the biofilm matrix [24]. Coating catheters with lysostaphin prevents catheter colonization [25]. Conflikt® (Decon) detergent disinfectant is a quaternary ammonium based disinfectant. Lysostaphin and Conflikt® were effective against biofilms at much lower doses than the antibiotics (Figs. 1, 2). The AB MBIC for ATCC 29213 with lysostaphin, for example, was 4 μg/ml (Fig. 1A), and for Conflikt® was 0.625% (Fig. 2A). Planktonic MICs for the four strains ranged from 2-4 μg/ml for lysostaphin, and from 0.039-0.078% for Conflikt®.
To help validate the described AB method for S. aureus biofilm susceptibility, AB assays were performed in parallel with XTT reduction assays, and CFU/ml were obtained from the same wells as AB absorbances. Reduction of tetrazolium salts, for e.g. XTT, is a common method of determining microbial cell viability [26], but XTT has several drawbacks, including toxicity. Alamar blue is stable and nontoxic, so unlike other metabolic assays, it is possible to plate directly out of wells over long periods for determination of viable cell counts. Similarly, in the CLSI planktonic assay [22], minimum bactericidal concentrations (MBCs) are obtained from the same wells as MICs. Biofilms of S. aureus ATCC 29213 and three clinical isolates were established overnight and treated with lysostaphin or Conflikt® for 20 h. Alamar blue or XTT/menadione was added, and after 1 h, absorbances were obtained and wells scraped for dilution-plating (Figs. 1, 2). There was typically a small amount of biofilm growth from 24 h (Figs. 1, 2 arrows) to 44 h (Figs. 1, 2, 0 μg/ml). As evident in Figures 1 and 2, the AB MBIC corresponds to a many log-fold reduction in CFU/ml. These results support the hypothesis that metabolic activity is a useful measurement of viability, and that the AB assay is an extremely sensitive method.
Pearson's two-tailed correlation coefficients were calculated from the data in Figures 1 and 2. With the exception of Conflikt®-treated S. aureus 520020, where AB vs. XTT and AB vs. CFU/ml were moderately correlated (r = 0.76, r = 0.81, respectively) [Table 2], AB had excellent correlation with XTT and CFU/ml (Table 2).
Discussion
Together, S. aureus and S. epidermidis are responsible for most implant and prosthetic device infections. As such, development of a standardized method to assess drug susceptibility of biofilms from both species is critical. An automated microtiter plate assay was recently described for susceptibility testing of S. aureus biofilms [27]. However, the assay was based on crystal violet staining, which does not measure viability. A high-throughput method employing AB, which does measure viability, has been described for S. epidermidis [14]. Recently, Peeters et al. [26] compared a variety of methods for quantifying biofilms (no susceptibility testing), and found the fluorescent AB assay to be very reliable for quantifying S. aureus biofilms. Alamar blue reduction data can be collected with the naked eye, or for increased sensitivity, with either fluorescence-based or absorbance-based instruments. Because absorbance-based instruments can be found in most laboratories, we evaluated the applicability of the AB method for susceptibility testing of S. aureus biofilms using absorbance. The biofilms produced by the four S. aureus isolates were so resistant to conventional antibiotics that lysostaphin and Conflikt® had to be employed for correlation studies.
Although biofilm structure, growth and regulation in S. aureus and S. epidermidis is not equivalent [16–18], we have demonstrated that the AB assay is a reliable, reproducible method of evaluating biofilm drug susceptibility for both species [present study and [14]]. The standardization and correlation results presented here suggest that this method has promise for S. aureus biofilm susceptibility testing, but evaluation against a large panel of S. aureus strains is warranted. Certainly, it would be useful to have an effective conventional antibiotic for these larger studies, instead of widely reactive enzymes and detergents. Should AB be developed as a standard method of biofilm susceptibility testing, it would be useful to have consensus on the definition of the MBIC. While this and a previous study [14] used a value of ≤ 50% reduction of AB (which, as demonstrated, corresponds to a many log-fold reduction in CFU), much more data from a variety of labs will be necessary to define the most broadly applicable cutoff.
The benefits of AB over other methods of biofilm susceptibility testing are numerous and include simplicity, relative cost, compatibility with high throughput, lack of toxicity and importantly, AB measures viability, not simply mass. The major benefit of AB over XTT/menadione is its lack of toxicity (menadione has human toxicity), and the obvious benefit of AB over CFU/ml determination is its simplicity. The AB biofilm assay can be performed in any lab with a spectrophotometer, and is an excellent choice for high throughput labs. A standardized biofilm AB assay should greatly increase the rate of discovery of Staphylococcus biofilm specific agents.
References
Schierholz JM, Beuth J: Implant infections: a haven for opportunistic bacteria. J Hosp Infect. 2001, 49: 87-93. 10.1053/jhin.2001.1052
Stamm WE: Prevention of infections. Infections related to medical devices. Ann Intern Med. 1978, 89: 764-69.
Maki DG: Infections due to infusion therapy. Hospital Infections. Edited by: Bennett JV, Brachman PS. 1992, 849-898. Boston, MA: Little, Brown and Co.
Moreillon P, Que YA: Infective endocarditis. Lancet. 2004, 363: 139-49. 10.1016/S0140-6736(03)15266-X
Waldvogel FA, Bisno AL: Infections associated with indwelling medical. 2000
Zimmerli W, Trampuz A, Ochsner PE: Prosthetic-joint infections. N Eng J Med. 2004, 351: 1645-54. 10.1056/NEJMra040181.
Donlan RM, Costerton JW: Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002, 15: 167-93. 10.1128/CMR.15.2.167-193.2002
Stewart PS, Mukherjee PK, Ghannoum MA: Biofilm antimicrobial resistance. Microbial Biofilms. Edited by: Ghannoum M, O'Toole GA. 2004, 250-268. Washington DC: ASM Press.
Amorena B, Gracia E, Monzón M, Leiva J, Oteiza C, Pérez M, Alabart J, Hernández-Yago J: Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J Antimicrob Chemother. 1999, 44: 43-55. 10.1093/jac/44.1.43
Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A: The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999, 37: 1771-76.
Hodson AE, Nelson SM, Brown M, Gilbert P: A simple in vitro model for growth control of bacterial biofilms. J Appl Bacteriol. 1995, 79: 87-93.
Kharazmi A, Giwercman B, Holby N: Robbins device in biofilm research. Meth Enzym. 1999, 310: 207-215. full_text
Polonio RE, Mermel LA, Paquette GE, Sperry JF: Eradication of biofilm-forming Staphylococcus epidermidis (RP62A) by a combination of sodium salicylate and vancomycin. Antimicrob Agents Chemother. 2001, 45: 3262-66. 10.1128/AAC.45.11.3262-3266.2001
Pettit RK, Weber CA, Kean MJ, Hoffman H, Pettit GR, Tan R, Franks KS, Horton ML: Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob Agents Chem. 2005, 49: 2612-17. 10.1128/AAC.49.7.2612-2617.2005.
Repp KK, Menor SA, Pettit RK: Microplate Alamar blue assay for susceptibility testing of Candida albicans biofilms. Med Mycol. 2007, 45: 603-07. 10.1080/13693780701581458
Cassat JE, Lee CY, Smeltzer MS: Investigation of biofilm formation in clinical isolates of Staphylococcus aureus. Methods in Molecular Biology. Edited by: Kropsinski AM, Totowa NJ. 2007, 127-144. Humana Press,
Izano EA, Amarante MA, Kher WB, Kaplan JB: Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008, 74: 470-76. 10.1128/AEM.02073-07
O'Gara JP: ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 2007, 270: 179-88. 10.1111/j.1574-6968.2007.00688.x
Hawser SP, Douglas J: Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect Immun. 1994, 62: 915-21.
Kuhn DM, Chandra J, Mukherjee PK, Ghannoum MA: Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun. 2002, 70: 878-88. 10.1128/IAI.70.2.878-888.2002
Kuhn DM, Balkis M, Chandra J, Mukherjee PK, Ghannoum MA: Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J Clin. 2003, 41: 506-08.
Clinical and Laboratory Standards Institute: Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, Approved standard M7-A5. 2000, Villanova, PA: NCCLS, 4.
Schindler C, Schuhardt V: Lysostaphin: a new bacteriolytic agent for the Staphylococcus. Proc Natl Acad Sci USA. 1964, 51: 414-21. 10.1073/pnas.51.3.414
Wu JA, Kusuma C, Mond JJ, Kokai-Kun JF: Lysostaphin disrupts Staphylococcus aureus and Staphylococcus epidermidis biofilms on artificial surfaces. Antimicrob Agents Chemother. 2003, 47: 3407-14. 10.1128/AAC.47.11.3407-3414.2003
Shah A, Mond J, Walsh S: Lysostaphin-coated catheters eradicate Staphylococcus aureus challenge and block surface colonization. Antimicrob Agents Chemother. 2004, 48: 2704-07. 10.1128/AAC.48.7.2704-2707.2004
Peeters E, Nelis HJ, Coenye T: Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Meth. 2008, 72: 157-65. 10.1016/j.mimet.2007.11.010.
Sandberg M, Määttänen A, Peltonen J, Vuorela PM, Fallarero A: Automating a 96-well microtitre plate model for Staphylococcus aureus biofilms: an approach to screening of natural antimicrobial compounds. Int J Antimicrob Agents. 2008, 32: 233-40. 10.1016/j.ijantimicag.2008.04.022
Acknowledgements
Financial support was provided by grants R01 CA90441-01-05, 2R56 CA090441-06A1, and 5R01 CA090441-07 from the Division of Cancer Treatment, Diagnosis and Centers, National Cancer Institute, DHHS, the Arizona Biomedical Research Commission, the Robert B. Dalton Endowment Fund and Dr. Alec Keith. We thank G. Cage, Arizona Department of Health Services, for providing clinical isolates of S. aureus.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RKP designed the studies, performed the statistical analyses, and wrote the manuscript. CAW performed the studies and participated in data analyses. GRP arranged financial support and critically evaluated the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Pettit, R.K., Weber, C.A. & Pettit, G.R. Application of a high throughput Alamar blue biofilm susceptibility assay to Staphylococcus aureus biofilms. Ann Clin Microbiol Antimicrob 8, 28 (2009). https://doi.org/10.1186/1476-0711-8-28
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-0711-8-28