Abstract
Background
Life trauma is highly prevalent in the general population and posttraumatic stress disorder is among the most prevalent psychiatric consequences of trauma exposure. Brazil has a unique environment to conduct translational research about psychological trauma and posttraumatic stress disorder, since urban violence became a Brazilian phenomenon, being particularly related to the rapid population growth of its cities. This research involves three case-control studies: a neuropsychological, a structural neuroimaging and a molecular neuroimaging study, each focusing on different objectives but providing complementary information. First, it aims to examine cognitive functioning of PTSD subjects and its relationships with symptomatology. The second objective is to evaluate neurostructural integrity of orbitofrontal cortex and hippocampus in PTSD subjects. The third aim is to evaluate if patients with PTSD have decreased dopamine transporter density in the basal ganglia as compared to resilient controls subjects. This paper shows the research rationale and design for these three case-control studies.
Methods and design
Cases and controls will be identified through an epidemiologic survey conducted in the city of São Paulo. Subjects exposed to traumatic life experiences resulting in posttraumatic stress disorder (cases) will be compared to resilient victims of traumatic life experiences without PTSD (controls) aiming to identify biological variables that might protect or predispose to PTSD. In the neuropsychological case-control study, 100 patients with PTSD, will be compared with 100 victims of trauma without posttraumatic stress disorder, age- and sex-matched controls. Similarly, 50 cases and 50 controls will be enrolled for the structural study and 25 cases and 25 controls in the functional neuroimaging study. All individuals from the three studies will complete psychometrics and a structured clinical interview (the Structured Clinical Interview for DSM-IV and the Clinician-Administered PTSD Scale, Beck Anxiety Inventory, Beck Depression Inventory, Global Assessment of Function, The Social Adjustment Scale, Medical Outcomes Study 36-Item Short-Form Health Survey, Early Trauma Inventory, Clinical global Impressions, and Peritraumatic Dissociative Experiences Questionnaire). A broad neuropsychological battery will be administered for all participants of the neuropsychological study. Magnetic resonance scans will be performed to acquire structural neuroimaging data. Single photon emission computerized tomography with [(99m)Tc]-TRODAT-1 brain scans will be performed to evaluate dopamine transporters.
Discussion
This study protocol will be informative for researchers and clinicians interested in considering, designing and/or conducting translational research in the field of trauma and posttraumatic stress disorder.
Similar content being viewed by others
Background
Posttraumatic stress disorder (PTSD) occurs following exposure to a potentially traumatic life event and is defined by three symptom clusters: reexperiencing, avoidance and numbing, and arousal [1]. However, PTSD is relatively rare event in trauma-exposed people. This fact has motivated research aimed at identifying risk factors for this disorder. Two meta-analyses of PTSD risk factors have come to some consensus as to the key factors influencing PTSD vulnerability. These include small but consistent effects on risk for pre-trauma factors such as cognitive ability, family psychiatric history, pre-trauma psychological adjustment, child abuse, other previous trauma exposures, and general childhood adversity [2, 3]. Characteristics of the traumatic experience were found to be particularly important, especially trauma severity, perceived life threat and peri-traumatic emotional reactions such as dissociation [2, 3]. A dose-response relation between severity of exposure and conditional risk of developing PTSD has been well-documented [4, 5]. Post-trauma social support also appears to play a role [2, 3]. However, the risk factors models supported by meta-analytic studies explain only about 20% of the variance in PTSD; clearly new variables need to be incorporated into models of PTSD vulnerability. Due in part to methodological limitations of extant research, the role of neuropsychological and brain structure and functional factors in the etiology of PTSD are less well understood.
This paper describes the protocol for the Project Post-Traumatic Stress Disorder in Brazil which is aimed at characterizing the underlying biology of PTSD neuropsychological assessment, neurostructural evaluation and molecular imaging of the dopamine transporter system. Brazil offers a unique environment to conduct translational research about psychological trauma and PTSD. From 1980 to 2000, a total of more than 598 thousand people died in Brazil because of homicide with two thirds of this occurring in the 90's. In 1980 the leading cause of violent death in the country was traffic accidents but in 2000 it was homicides [6, 7]. From 1991 to 2000 there was an increase of 27% in the proportion of deaths caused by homicides among the total deaths in the country. Thus, lethal violence became a national phenomenon, being particularly related to the rapid population growth of large and urban cities [8].
Neuropsychological findings in PTSD
PTSD is characterized by re-experiencing of the traumatic event and the inability to consciously recall facts about the traumatic event as well as by altered emotional processing of trauma-relevant cues. The patient's memory seems to be fixed on the traumatic event, and the retrieval of others memories seems to be inhibited [9–11]. Previous research on the neuropsychology of PTSD has identified several neurocognitive deficits [12]. Neuropsychological measures of intellectual ability, learning, memory (verbal and non-verbal), attention, visuospatial ability, executive functioning, language, psychomotor speed have been examined. Several studies have identified impaired performance on verbal memory and learning in PTSD cases as compared to controls [13–16]. Other studies have documented differences between cases and controls in different domains, including attention, working memory [17–22], and processing speed [21]. Although there is evidence for memory impairment in PTSD subjects, it remains unclear whether memory impairment is confined to verbal material or nonverbal material is also affected [11, 14, 21]. Different studies, have found impaired visual memory in individuals with PTSD [18, 19, 23, 24].
Many studies have shown impairments in the whole mnemonic process (immediate memory, recall and recovery), attention, learning, intellectual level, and emotional processing [25]. Nonetheless, others studies have shown no differences between cases and controls for memory [24, 26–29] and/or attention performance [12, 13, 30]. Those neuropsychological findings suggest involvement of two primary strcutures: hippocampus and prefrontal cortex. Several studies have reported decreases in hippocampal volume [31–36] and hippocampal N-acetylaspartate [37–39] as well as an association between hippocampal atrophy and poor verbal memory in PTSD subjects [23]. Neurofunctional studies have indicated specific findings in limbic regions, although the relationship of these results to neuropsychological performance remains to be explored [40].
An alternative model of PTSD may be related to a dysfunction of higher-level attentional resource which in turn might affect activity in other systems concerned with memory and thought [20, 41, 42]. Attention and concentration difficulties appear to be core deficits in PTSD and memory deficits might actually be secondary to an impaired attention. A possible explanation for the association between memory and attention difficulties in PTSD is that explicit memory performance may be impacted by impaired attention resources during processing. Some researchers have theorized that the heightened emotional reactivity in patients with PTSD disrupts attentional resources [43]. Impaired attention prevents sufficient registration of information, which in turn prevents consolidation and retrieval of memory. Furthermore, recent studies have demonstrated a possible deficit in the inhibitory processes of memory in PTSD [18, 19, 44].
Several potential confounding factors must be considered for neuropsychological evaluation of PTSD patients including comorbid depression, substance abuse, and medical conditions, type of trauma, and motivational aspects. Therefore, any conclusion if deficits observed are specifically related to PTSD should take into account alternative explanations such as the possible effect of confounding factors [26, 40]. In conclusion, neuropsychological functioning has emerged as promising endophenotype to be explored in PTSD.
Neurostructural findings in PTSD
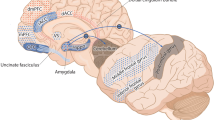
One of the possible mechanisms underlying the physiopathology of PTSD is the damaging action of glucocorticoids on hippocampus [45]. The hippocampus plays a central role in neuropsychological functions such as memory and emotional behaviour and is likely to be involved in the development of PTSD. An alternative model would be a failure in the regulatory activity of prefrontal cortex over amygdala, with a consequent hyperactivity of the later in response to traumatic memories [46–48]. This inhibitory deficiency is also a possible explanation for the hyperexcitability symptoms found in PTSD. The orbitofrontal cortex, the ventral portion of prefrontal cortex, appears to be central in the regulation of prefrontal cortex over amygdala and is also implicated in the processing of negative emotion [49]. Studies using different techniques have consistently found neurofunctional abnormalities in this region in PTSD patients [50–52]. Furthermore, some symptoms that may be seen in PTSD, such as poor impulse control and violent assaults, are also reported in individuals with lesions in orbitofrontal cortex [53, 54]. Findings from magnetic resonance imaging studies have suggested neurostructural alterations in PTSD. Although these findings are less consistent than those seen in neurofunctional studies, volumetric reductions in hippocampus [23, 32, 34–36, 51, 52] and prefrontal regions [55, 56] have been reliably found. However, additional studies are still warranted in order to assess whether these structural abnormalities are specific to PTSD or represent non-specific morphological abnormalities associated to trauma exposure. Further examination of abnormalities in frontolimbic structures are also required to clarify the role of structural and functional brain abnormalities in the pathophysiology of PTSD.
Molecular imaging of dopamine transporter in PTSD
Physiological response to stressful experience involves a vast neural-endocrine-immunologic reaction that leads to the release of catecholamines and autonomic nervous system stimulation [57]. Noradrenergic and hypothalamus-pituitary-adrenal axis systems are the most common studied in response to stress [58] but many other neural-chemical systems are implicated. In animal studies, dopaminergic innervation of the basolateral nucleus of the amygdala, the medial prefrontal cortex and other limbic regions is highly responsive to stress and may be altered by stress [59–61]. Also the enhancement of the acoustic startle response, which can be a symptom of PTSD, has been related to the dopamine D1 receptor agonists in rats [62]. Genetically determined alterations in dopamine release and dopamine receptor expression in mice have been implicated in behavioral abnormalities induced by chronic stress [63]. This finding was interpreted as suggesting that stress-induced alterations of central dopaminergic neurotransmission may be genotype-dependent and expressed in behaviour. Human studies showed that there was a relationship between urinary excretion of dopamine and plasma dopamine and (the severity of) PTSD symptoms [64].
Evidence from genetic studies has proposed that reduced density of D2 dopaminergic receptor predisposes to PTSD [65]. There are two important PTSD candidate genes that directly affect the dopamine system: the dopamine receptor gene (DRD2) and the dopamine transporter (DAT) gene. The D2 dopamine receptor (DRD2) minor (A1) allele DRD2 A1 has already been linked to ADHD, Tourette's syndrome, conduct disorder and substance abuse [66]. This prompted suppositions that this gene may be involved in stress response in humans [61]. Polymorphism of the dopamine transporter (DAT) gene, in the locus SLC6A3 3' (VNTR), has been found to predispose to PTSD and to chronic forms of the disorder [67]. Taken together, these evidences suggest a relevant role for dopamine in the pathogenesis of PTSD.
To gain more clarity about any link between PTSD and the DAT, it is therefore important to clearly document PTSD patients, controlling confounders as alcohol consumption, major depression and clinical illness, through a functional neuroimaging investigation.
Although molecular imaging allows reliable information on in vivo dopaminergic function [68], no studies, to our knowledge, has examined dopaminergic system activity in PTSD patients using molecular neuroimaging techniques.
The main reasons that justify such an effort to understand the problem of violence and its consequences for mental health can be described as follows: 1) Treatment strategies to be sponsored by the Brazilian public health care system need to be based on solid local data on the extent and nature of the disorder; 2) Exposure to traumatic life events is related to not only with mental disorders which include PTSD and depression, but also with other cognitive and neurotransmission dysfunctions [18, 19, 44, 69]. The impact in mental health of the population of exposure to violence among the population of Sao Paulo, a large urban centre in a Middle Income Country (MIC), as well as specific parameters such as neurocognitive, neurostructural anf functional neuroimaging finds is virtually unknown.
The subjects of the study will be selected from an epidemiological/genetic survey in the city of Sao Paulo to assess the relationship between exposure to violence and the prevalence of PTSD and common mental disorders. Subjects located in the epidemiological/genetic survey will be referred to these three case-control studies, reported here, and to a randomized controlled clinical trial on the efficacy of topiramate for the treatment of PTSD symptoms.
This protocol is the result of collaborative task force to conduct translational research in the field of traumatic stress in urban regions of Brazil. The current project investigates possible causes for neurocognitive deficits, neurostructural changes and dopaminergic dysfunction in individuals with PTSD. We will be comparing individuals with current or lifetime diagnosis of PTSD with those who were exposed to a traumatic event but did not develop a current or a lifetime diagnosis of PTSD.
The main objectives of this project are:
1. to examine cognitive functioning of PTSD subjects and its relationships with symptomatology;
2. to evaluate neurostructural integrity of orbitofrontal cortex and hippocampus in PTSD subjects;
3. to evaluate if patients with PTSD have decreased dopamine transporter density in the basal ganglia as compared to resilient controls subjects.
Methods and design
Sample
Cases and controls will be identified through an epidemiologic survey conducted in the city of São Paulo. Details of the epidemiologic study are presented in a companion paper (cite) and are summarized here. To identify trauma victims in the community, interviews were conducted by a professional team specialized in household surveys, the Brazilian Institute of Public Opinion and Statistics. Interviewers were trained at the CIDI [70] in the Federal University of São Paulo, an accredited center by the World Health Organization (WHO). Training procedures were conducted in accordance to the guidelines set up by the WHO. Interviews were carried out in the participants households by means of printed questionnaires. All questionnaires were translated into Portuguese and adapted to the local social and cultural context. Inclusion and exclusion criteria for the three case-control studies are described in tables 1 and 2, respectively. Individuals who met inclusion criteria during the epidemiologic study were invited to participate in the case-control study. Subjects exposed to traumatic life experiences resulting in PTSD (cases) will be compared to resilient subjects victims of traumatic life experiences without PTSD (controls) aiming to identify biological variables that might protect or predispose to PTSD. This case-control design will enrol representative subjects from the community being this procedure an important technique to overcome Berkson bias. Subjects will be informed about the procedures of the studies and will be asked to formally consent willingness to participate. Subjects who consent to participate in the case-control studies will receive the following assessment:
Measures
Clinical and Demographic assessment
1) Sociodemographic data will be obtained based by using an adapted form of the CIDI sociodemographic section;
2) Structured Clinical Interview for DSM-IV (SCID) I: SCID is a semi structured interview for the DSM-IV [71, 72]. It allows the diagnosis of mental health disorders according to DSM IV criteria and has already been validated for Brazilian population [73];
3) Clinician Administered PTSD Scale (CAPS) [74]: A clinician rating scale for assessing current and lifetime PTSD: the CAPS-1. CAPS is a structured clinical interview designed to be applied by clinician and its validation was included as part of the first phase of this protocol. It is a 30 items scale investigating the frequency and intensity of PTSD symptoms and traumatic life experiences.
4) Beck Anxiety Inventory (BAI): BAI is a self-administered 21 items questionnaire assessing intensity of anxiety symptoms [75];
5) Beck Depression Inventory (BDI) is used to assess depressive symptoms in clinical settings [108]; it is a self-administered 21 items questionnaire, and it has been validated for the Brazilian population [76];
6) Global Assessment of Function (GAF) scale provides data on the clinical global state of patients [77];
7) The Social Adjustment Scale (SAS) is a self-administered instrument to assess social adaptation [78, 79] and it has been validated to the Brazilian social and cultural context [80];
8) Medical Outcomes Study 36-Item Short-Form Health Survey (MOS SF-36) [81] is a self-report scale constructed to collect data on health status, functioning, and well-being. A Portuguese version of the questionnaire has already been tested for its validity and reliability in Brazil [82];
9) Early Trauma Inventory (ETI) is a semi-structured interview comprising 56 items to measure traumatic life experiences occurred in early life, in the following domains: sexual, physical and psychological abuse and other traumatic life experiences [83];
10) Clinical global Impressions (CGI) is a scale to assess treatment response in patients with mental disorders [84];
11) Peritraumatic Dissociative Experiences Questionnaire (PDEQ) [85] is a reliable and valid measure of peritraumatic dissociation as previously described in the epidemiologic study section.
The Neuropsychological Assessment
The neuropsychological evaluation will be performed in a single session by neurophysiologists trained in the instruments listed as follows. The training has been conducted by a senior psychologist, acquainted to the assessments chosen for the study, who will be responsible for supervising the trainees in order to keep the accuracy of measurements. The following tests will be part of the Neuropsychological Assessment:
1) The Wisconsin Card Sorting Test (WCST) will be used to assess cognitive set shifting and executive functions [86];
2) Vocabulary and Blocks – Subscales WAIS III is a widely used measure for intellectual level[87];
3) The Digit Span – Subscale WAIS III is an important tool for evaluating working memory and short-term memory[87];
4) The Spatial Span – Subscale WMS III is meant for assessment of immediate nonverbal memory and nonverbal working memory[87];
5) The International Affective Picture System (IAPS) was validated in Brazil and measures visual memory and emotional reaction through positive, negative and neutral figures [87];
6) The Rey Auditory-Verbal Learning Test (RAVLT) assesses the verbal learning and memory [88]
7) The Stroop Test is designed to assess selective attention and cognitive flexibility[89]
8) The Visual Reproduction – Subscale WMS III assess visual memory[87];
9) Cancellation of Mensulan assesses selective visual attention, vigilance and visual neglect[90];
10) Social and Occupational Functioning Assessment Scale (SOFAS) [1] – Assess the social and occupational functioning in the community.
Magnetic Resonance Imaging
Imaging data will be acquired at the Instituto do Sono, Federal University of Sao Paulo, using a GE1.5-T Signa scanner. Structural MR images will be acquired using a sagittal T1 acquisition series (TR = 9.8 ms, TE = 3.1 ms, flip angle = 30°, NEX = 1, matrix size = 256 × 256, FOV = 24 cm, thickness = 1.0 mm). A T2-weighted image series will also be acquired. Before scanning, a sagittal scout series (nine to eleven 5-mm-thick slices with a 1-mm interslice gap) will be performed to determine image quality and clarity as well as subject head position. Measurements will be conducted on PC workstation with the aid of BRAINS2 software [91]. Before tracing, T1- and T2-weighted images will be spatially realigned so that the brain anterior-posterior axis is parallel to the intercommissural line, which was horizontal in the sagittal plane, and the interhemispheric fissure is vertical in the axial plane. Six brain-limiting points (anterior, posterior, superior, inferior, left, and right) will then be picked to place images into the standard Talairach three-dimensional space [92]. After coregistering and fitting the three image sequences, a multimodal tissue classification will be performed using a Bayesian classifier based on discriminant analysis. This segmentation method automatically generates thresholds permitting the discrimination of grey and white matter as well as cerebrospinal fluid.
Hippocampus
The hippocampus will be traced manually on the coronal plane as described by Pantel et al [93]. Tracings begin with the generation of auxiliary guideline traces on the sagittal plane. The auxiliary traces are necessary to provide a neuroanatomically correct separation of rostral and caudal parts of the hippocampus from adjacent nonhippocampal brain tissue. Tracing will begin on the most medial slices. The starting slice is identified by choosing the slices that (going from medial to lateral) first show the cerebral peduncle separated from the upper pons. Once the anterior border of the hippocampus is identified on the starting slice, the vertical crosshairs will be placed anteriorly to this border. This procedure facilitates the identification of the anterior border on the following slices, because the head of the hippocampus, in general, does not extend beyond this level on the more lateral slices. The anterior border is outlined by the alveus and the uncal recess, which may be obliterated. Dorsally, CSF of the temporal horn of the lateral ventricle outlines the body, whereas the pulvinar thalamus serves as the border for the tail. On the medial slices the body is bordered by the fimbria, which is excluded from the trace itself. The posterior border is formed by the CSF of the lateral ventricle. The ventral border is defined by the WM of the temporal lobe.
Orbitofrontal cortex
The orbitofrontal cortex (OFC) will be outlined according to the proposed geometrical method developed by Lacerda et al [49]. The OFC will be manually measured in the coronal plane. The tip of the genu of corpus callosum will be located in the sagittal plane and used as the most posterior slice to be traced in the coronal plane. The last slice traced will be the most anterior coronal slice where brain tissue can be identified. The superior limit will be divided in two parts to reflect more the actual anatomical boundary of the OFC. In the subgenual regions, and specifically from the tip of the genu to the most anterior part of the CC, the superior boundary will be represented by the inferior border of the anterior cingulate corresponding to a midpoint at the interhemispheric fissure about five slices (5.08 mm) below the intercommissural line. More anteriorly, and specifically in the slices ahead of the genu of the CC, the superior limit will be represented by a midpoint placed on the intercommissural line. This "lowering" of the superior limit will be done to avoid inclusion of subgenual structures in the first slices that are not traditionally considered to be part of OFC (e.g., anterior cingulate).
In all slices, horizontal and vertical crosshairs will be placed as tangent lines at the inferior and lateral surfaces of the frontal lobes, respectively. The intersection of these two lines (horizontal and vertical crosshairs) will generate two lateral points that will be connected to the superior limit point, composing the lateral boundaries of the tracings. The inferior border will be traced following the inferior surface of the frontal lobes between the two lateral boundaries described above. The OFC will also be subdivided into gyrus rectus and orbital gyri by tracing a line through the olfactory sulcus. This subdivision will not be conducted in the most anterior slices where the olfactory sulcus disappears.
SPECT scans of Dopamine Transporter
The kits of TRODAT-1 were obtained through a scientific collaboration with the Research Institute of Nuclear Energy, Lung-Tan, Taiwan. The metastable technetium-99 was produced by a generator of [99Mo] (molybdenum-99) from the Institute of Nuclear Energy Research (IPEN-SP) with freshly elution. The kits of TRODAT-1 were marked with [99mTc] according to the technique developed by Kung et al[94]. Sixty mCi of elution of Sodium Pertechnetate [99mTc] diluted in 5 ml of saline solution are injected in the kit and submitted to 16 atmospheres and temperature of 120°C, during 30 minutes in an autoclave. Later, the solution of [99mTc] TRODAT-1 is cooled at room temperature.
The images will be acquired through a single photon emission computerized tomography (SPECT) Gama camera of the type (Hawkeye General Electric Medical System, USA) according to the methodology previously validated [95]. All subjects will receive an intravenous injection of 2 ml containing among 22 to 25 mCi of [99mTc]-TRODAT-1 in an antecubital peripheral vein. Images will be acquired 4 hours after the injection. The SPECT modality of the system with two heads and fan-beam collimators of ultra-high resolution will be used. Energy Window will be 140 ± 14 keV and matrix of 128 × 128 in circular orbit with step and shoot movements of 64 steps for each head will be used, with diameter and degree of rotation of 30 cm and 360°, respectively. The time of acquisition for the projection will be of 20 seconds, with a factor of zoom of 1.45. The reconstruction of the SPECT images will be accomplished through a filter algorithm of filtered retroprojection and a Butterworth filter of 0.4 cut off with pixels of 10th order. Three-dimensional images of the whole brain will be obtained and, for the analysis, two transaxial slices will be used at the level of the striatal body, with 3 mm of thickness corresponding to the level of the largest captation of the radiotracer. DAT density will be calculated with binding potential (DAT-BP) using regions of interests (ROI) bilaterally drawn in the striatum (STR) and the occipital cortex (OCC-background). BP will be calculated with the formula striatum (STR-OCC)/OCC.
Data management and Analyses
-
1)
Questionnaires will be double typed and data will be entered on SPSS software data files. Participants will be divided into two categories, according to the diagnosis: (1) lifetime diagnosis of PTSD and (2) Traumatic experience, but no lifetime diagnosis of PTSD. The following comparisons will be carried out in the two groups:
-
1)
Neuropsychological Study: neuropsychological measures of PTSD cases compared to controls;
-
2)
Magnetic resonance imaging study: to assess neurostructural abnormalities in OFC and hippocampus of patients with PTSD compared to resilient controls;
-
3)
Molecular neuroimaging study: dopamine transporter density using single photon emission tomography of PTSD cases compared to controls.
Data will be codified and analyzed using the Statistical Package for Social Sciences (SPSS for Windows, version 15.0). Proportion differences will be compared using the Chi Square test or Fisher's exact test, as appropriate. Continuous variables will be compared by Analysis of Variance (ANOVA) or Mann-Whitney test for non-parametric data. All significant tests will be considered as 2-tailed. P values < 0.05 will be considered statistically significant. Because of the exploratory nature of the study, we do not consider alpha adjustment in multiple comparisons and we did not calculate the sample size (Table 3). The main analyses will examine the relationship between exposure to traumatic events and the PTSD occurrence. Unadjusted relative risk for studied variables cognition, cortisol level, hippocampal volume, and dopamine transporter density (and 95% confidence intervals) are presented for trauma without PTSD and trauma with PTSD, both with and without adjustment for age (18–29 years, 30–39 years, 40–49 years, 50–60) and gender. As a secondary analysis, we will be adjusting for clinical and demographic variables previously associated with PTSD: marital status, country of birth, socio-economic status, urbanicity and employment status.
Ethical Issues
Participants will be informed about research procedures and risks and signed an informed consent submitted and approved by the Ethical Committee of the Federal University of São Paulo (Processes: 1-Neuropsychology-0124/06; Neurostructural-1026/06; Molecular neuroimaging-0295/06). Subjects diagnosed as having any mental health disorder will be offered a referral to the out-patient clinic at the Federal University of Sao Paulo.
Discussion
This study protocol illustrates a collaborative work of potential value to researchers interested in innovative and realistic investigation aimed at understanding the neurobiology of PTSD in a population-representative sample. This article describes the methodological responses to challenges in conducting translational research, where patients are identified from a real-world scenario in a population-based study and go through neuropsychological, neurostructural and molecular neuroimaging techniques.
A recent review of the literature found over 60 studies examining memory and attention performance in PTSD subjects, but few studies have examined learning, executive functioning, and emotional reaction in this population. A key aim of the present neuropsychological study is not only to assess those less explored domains but, also, try to clarify some discrepancies reported in literature by examining a more homogeneous, adequately controlled sample. Some inconsistencies in neuropsychological findings may be attributed at least in part to sample limitations. Neuropsychological deficits involving attention and memory have been replicated in different samples including war veterans [14, 15, 20], rape victims, and other traumatized populations. However, most neuropsychological studies of PTSD involve war veterans with chronic PTSD who frequently exhibit comorbid psychiatric conditions such as depression, anxiety and substance use disorders, which represent major confounders [15]. The effects of depressed mood on neuropsychological functioning have been well documented [96]. The high rate of comorbidity and overlap of symptoms between these two disorders, however, makes it difficult to exclude individuals with current depression from PTSD studies. Therefore, it is important to address the presence of comorbid depression and to include measures of depression as covariates in analyses.
Despite the unquestionable progress in identification of neurocognitve and neuroanatomical abnormalities associated with PTSD over the past decade or so, it remains unclear whether neurostructural and neuropsychological alterations are specific to PTSD or are related to unspecific environmental factors such as stress and substance abuse. The findings from this neuropsychological study, together with data from neurostructural investigation, may offer an uncommon opportunity to examine the convergence of cognitive and neuroanatomical alterations in patients with PTSD. Stress-induced functional and structural alterations in hippocampus and OFC may mediate many of the symptoms of PTSD that are related to memory dysregulation and hyperexcitability. Interestingly, reversion of both neuropsychological and neuroanatomical abnormalities has been demonstrated after treatment with paroxetine, which in turn has been shown to promote neurogenesis in animal studies [97]. The increasing use of sophisticated neuroimaging techniques is certainly enhancing our understanding of PTSD, potentially improving prevention, treatment, and cognitive rehabilitation programs [23].
Although a previous study showed involvement of DAT gene in PTSD [67], and other indirect investigations have suggested a connection between dopaminergic system and stress [61], insufficient research has been done on the role of the DAT in relation to PTSD. To the best of our knowledge this will be the first SPECT study investigating dopamine transporter density in patients with PTSD and well matched resilient controls coming from an epidemiologic sample. The results of this study will help to disentangle whether possible dopaminergic changes in PTSD are a "state condition" or a "trait" marker of this disorder, raising a discussion why some subjects exposed to trauma develop PTSD and some others do not. Moreover, further studies evaluating patients with PTSD, resilient controls and healthy control subjects (who never experienced trauma) would provide useful information to clarify whether dopaminergic abnormalities are related to PTSD itself or to psychological trauma exposure.
Advances in molecular imaging techniques, such as SPECT, have made important contributions to the understanding of the pathophysiology of neuropsychiatric disorders [98]. Molecular imaging approaches are more sensitive than Neuroanatomical imaging techniques, and are able to identify subtle cerebral pathophysiological changes before neurostructural abnormalities take place. One of the major goals of molecular imaging research has been the identification of biomarkers, which are defined as the characteristics that are objectively measured and can differentiate normal biologic processes from pathogenic processes. These approaches has the potential to provide accurate and early neuropsychiatric recognition, evaluate disease progression, and monitor treatment efficacy [68].
These studies have several important limitations. First, cases are recruited after they have developed PTSD. Assuming we find differences between cases and controls on neuropsychological/imaging variables, we will not be able to determine whether these differences reflect a risk factor or a consequence of the disorder. Second, in several situations both cases and controls, were exposed to traumatic experience years before the investigations, producing a potential recall bias.
This study protocol intends to be helpful for researchers and clinicians interested in designing and/or conducting translational research in the field of trauma and posttraumatic stress disorder.
References
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders – DSM-IV. 2005, Washington, D.C.: American Psychiatric Association, Fourth
Brewin CR, Andrews B, Valentine JD: Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000, 68: 748-766. 10.1037/0022-006X.68.5.748.
Ozer EJ, Best SR, Lipsey TL, Weiss DS: Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003, 129: 52-73. 10.1037/0033-2909.129.1.52.
Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P: Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998, 55: 626-632. 10.1001/archpsyc.55.7.626.
Kulka R, Schlenger W, Fairbank J, Hough R, Jordan K, Marmar C, et al: Trauma and the Vietnam War Generation: Report of the findings from the National Vietnam Veterans Readjustment Study. 1990, New York: Brunner/Mazel
Gawryszeski VP: Injury mortality report for São Paulo State, 2003. Sao Paulo Med J. 2007, 125: 139-143. 10.1590/S1516-31802007000300003.
Gawryszewski VP, Rodrigues EM: The burden of injury in Brazil, 2003. Sao Paulo Med J. 2006, 124: 208-213. 10.1590/S1516-31802006000400007.
UNESCO: Mapa da Violência. 1988, Brasilia: UNESCO
Cottencin O, Vaiva G, Huron C, Devos P, Ducrocq F, Jouvent R, et al: Directed forgetting in PTSD: a comparative study versus normal controls. J Psychiatr Res. 2006, 40: 70-80. 10.1016/j.jpsychires.2005.04.001.
Wessa M, Jatzko A, Flor H: Retrieval and emotional processing of traumatic memories in posttraumatic stress disorder: peripheral and central correlates. Neuropsychologia. 2006, 44: 1683-1696. 10.1016/j.neuropsychologia.2006.03.024.
Jelinek L, Jacobsen D, Kellner M, Larbig F, Biesold KH, Barre K, et al: Verbal and nonverbal memory functioning in posttraumatic stress disorder (PTSD). J Clin Exp Neuropsychol. 2006, 28: 940-948. 10.1080/13803390591004347.
Twamley EW, Hami S, Stein MB: Neuropsychological function in college students with and without posttraumatic stress disorder. Psychiatry Res. 2004, 126: 265-274. 10.1016/j.psychres.2004.01.008.
Yehuda R, Keefe RS, Harvey PD, Levengood RA, Gerber DK, Geni J, et al: Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1995, 152: 137-139.
Uddo M, Vasterling JJ, Brailey K, Sutker PB: Memory and attention in combat-related pos-traumatic stress disorder (PTSD). Journal of Psychopathology and Behavioral Assessment. 1993, 15: 43-52. 10.1007/BF00964322.
Samuelson KW, Neylan TC, Metzler TJ, Lenoci M, Rothlind J, Henn-Haase C, et al: Neuropsychological functioning in posttraumatic stress disorder and alcohol abuse. Neuropsychology. 2006, 20: 716-726. 10.1037/0894-4105.20.6.716.
Gil T, Calev A, Greenberg D, Kugelmass S, Lerer B: Cognitive functioning in post-traumatic stress disorder. Journal of Traumatic Stress. 1990, 14: 29-45. 10.1002/jts.2490030104.
Gilbertson MW, Gurvits TV, Lasko NB, Orr SP, Pitman RK: Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J Trauma Stress. 2001, 14: 413-432. 10.1023/A:1011181305501.
Vasterling JJ, Brailey K, Constans JI, Sutker PB: Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998, 12: 125-133. 10.1037/0894-4105.12.1.125.
Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Sutker PB: Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002, 16: 5-14. 10.1037/0894-4105.16.1.5.
Koso M, Hansen S: Executive function and memory in posttraumatic stress disorder: a study of Bosnian war veterans. Eur Psychiatry. 2006, 21: 167-173. 10.1016/j.eurpsy.2005.06.004.
Brandes D, Ben-Schachar G, Gilboa A, Bonne O, Freedman S, Shalev AY: PTSD symptoms and cognitive performance in recent trauma survivors. Psychiatry Res. 2002, 110: 231-238. 10.1016/S0165-1781(02)00125-7.
Beckham JC, Crawford AL, Feldman ME: Trail making test performance in Vietnam combat veterans with and without posttraumatic stress disorder. J Trauma Stress. 1998, 11: 811-819. 10.1023/A:1024409903617.
Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995, 152: 973-981.
Zalewski C, Thompson W, Gottesman I: Comparison of Neuropsychological Test Performance in PTSD, Generalized Anxiety Disorder, and Control Vietnam Veterans. Assessment. 1994, 1: 133-142. 10.1177/1073191194001002003.
Souza SE, Sanches M, Malta SM, Lacerda AL, Bressan RA: Neuropsicologia e TEPT. Transtorno do Estresse Pós-Traumático: diagnóstico e tratamento. Edited by: Mello MF. 2006, Barueri: Editora Monole Ltda
Twamley EW, Hami S, Stein MB: Neuropsychological function in college students with and without posttraumatic stress disorder. Psychiatry Res. 2004, 126: 265-274. 10.1016/j.psychres.2004.01.008.
Crowell TA, Kieffer KM, Siders CA, Vanderploeg RD: Neuropsychological findings in combat-related posttraumatic stress disorder. Clin Neuropsychol. 2002, 16: 310-321.
Neylan TC, Lenoci M, Rothlind J, Metzler TJ, Schuff N, Du AT, et al: Attention, learning, and memory in posttraumatic stress disorder. J Trauma Stress. 2004, 17: 41-46. 10.1023/B:JOTS.0000014675.75686.ee.
Stein MB, Hanna C, Vaerum V, Koverola C: Memory functioning in adult women traumatized by childhood sexual abuse. J Trauma Stress. 1999, 12: 527-534. 10.1023/A:1024775222098.
Golier J, Yehuda R, Cornblatt B, Harvey P, Gerber D, Levengood R: Sustained attention in combat-related posttraumatic stress disorder. Integr Physiol Behav Sci. 1997, 32: 52-61. 10.1007/BF02688613.
Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995, 152: 973-981.
Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al: Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse – a preliminary report. Biol Psychiatry. 1997, 41: 23-32. 10.1016/S0006-3223(96)00162-X.
Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al: Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002, 5: 1242-1247. 10.1038/nn958.
Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, et al: Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996, 40: 1091-1099. 10.1016/S0006-3223(96)00229-6.
Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B: Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997, 27: 951-959. 10.1017/S0033291797005242.
Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, et al: Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry. 2002, 52: 119-125. 10.1016/S0006-3223(02)01359-8.
Freeman TW, Cardwell D, Karson CN, Komoroski RA: In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Med. 1998, 40: 66-71. 10.1002/mrm.1910400110.
Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, et al: Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001, 50: 952-959. 10.1016/S0006-3223(01)01245-8.
Schuff N, Marmar CR, Weiss DS, Neylan TC, Schoenfeld F, Fein G, et al: Reduced hippocampal volume and n-acetyl aspartate in posttraumatic stress disorder. Ann N Y Acad Sci. 1997, 821: 516-520. 10.1111/j.1749-6632.1997.tb48319.x.
Horner MD, Hamner MB: Neurocognitive functioning in posttraumatic stress disorder. Neuropsychol Rev. 2002, 12: 15-30. 10.1023/A:1015439106231.
Stein MB, Kennedy CM, Twamley EW: Neuropsychological function in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002, 52: 1079-1088. 10.1016/S0006-3223(02)01414-2.
Koenen KC, Driver KL, Oscar-Berman M, Wolfe J, Folsom S, Huang MT, et al: Measures of prefrontal system dysfunction in posttraumatic stress disorder. Brain Cogn. 2001, 45: 64-78. 10.1006/brcg.2000.1256.
Siegel HS: Gordon Memorial Lecture. Stress, strains and resistance. Br Poult Sci. 1995, 36: 3-22. 10.1080/00071669508417748.
Cottencin O, Vaiva G, Huron C, Devos P, Ducrocq F, Jouvent R, et al: Directed forgetting in PTSD: a comparative study versus normal controls. J Psychiatr Res. 2006, 40: 70-80. 10.1016/j.jpsychires.2005.04.001.
Sauro MD, Jorgensen RS, Pedlow CT: Stress, glucocorticoids, and memory: a meta-analytic review. Stress. 2003, 6: 235-245.
Phillips ML, Drevets WC, Rauch SL, Lane R: Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003, 54: 504-514. 10.1016/S0006-3223(03)00168-9.
Phillips ML, Drevets WC, Rauch SL, Lane R: Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003, 54: 515-528. 10.1016/S0006-3223(03)00171-9.
Liberzon I, Britton JC, Phan KL: Neural correlates of traumatic recall in posttraumatic stress disorder. Stress. 2003, 6: 151-156.
Lacerda AL, Hardan AY, Yorbik O, Keshavan MS: Measurement of the orbitofrontal cortex: a validation study of a new method. Neuroimage. 2003, 19: 665-673. 10.1016/S1053-8119(03)00137-X.
Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, et al: Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999, 156: 575-584.
Bremner JD, Innis RB, Ng CK, Staib LH, Salomon RM, Bronen RA, et al: Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 1997, 54: 246-254.
Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, et al: MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003, 160: 924-932. 10.1176/appi.ajp.160.5.924.
Blair RJ: Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. J Neurol Neurosurg Psychiatry. 2001, 71: 727-731. 10.1136/jnnp.71.6.727.
Brower MC, Price BH: Neuropsychiatry of frontal lobe dysfunction in violent and criminal behaviour: a critical review. J Neurol Neurosurg Psychiatry. 2001, 71: 720-726. 10.1136/jnnp.71.6.720.
De B, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, et al: Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002, 52: 1066-1078. 10.1016/S0006-3223(02)01459-2.
Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, et al: Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003, 14: 913-916. 10.1097/00001756-200305230-00002.
Ledoux JE, Muller J: Emotional memory and psychopathology. Philos Trans R Soc Lond B Biol Sci. 1997, 352: 1719-1726. 10.1098/rstb.1997.0154.
Claes SJ: Corticotropin-releasing hormone (CRH) in psychiatry: from stress to psychopathology. Ann Med. 2004, 36: 50-61. 10.1080/07853890310017044.
Inglis FM, Moghaddam B: Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999, 72: 1088-1094. 10.1046/j.1471-4159.1999.0721088.x.
Goldstein LE, Rasmusson AM, Bunney BS, Roth RH: Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996, 16: 4787-4798.
Broekman BF, Olff M, Boer F: The genetic background to PTSD. Neurosci Biobehav Rev. 2007, 31: 348-362. 10.1016/j.neubiorev.2006.10.001.
Meloni EG, Davis M: Enhancement of the acoustic startle response by dopamine agonists after 6-hydroxydopamine lesions of the substantia nigra pars compacta: corresponding changes in c-Fos expression in the caudate-putamen. Brain Res. 2000, 879: 93-104. 10.1016/S0006-8993(00)02753-0.
Puglisi-Allegra S, Cabib S: Psychopharmacology of dopamine: the contribution of comparative studies in inbred strains of mice. Prog Neurobiol. 1997, 51: 637-661. 10.1016/S0301-0082(97)00008-7.
Hamner MB, Diamond BI: Elevated plasma dopamine in posttraumatic stress disorder: a preliminary report. Biol Psychiatry. 1993, 33: 304-306. 10.1016/0006-3223(93)90302-T.
Comings DE, Muhleman D, Gysin R: Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: a study and replication. Biol Psychiatry. 1996, 40: 368-372. 10.1016/0006-3223(95)00519-6.
Noble EP: The DRD2 gene in psychiatric and neurological disorders and its phenotypes. Pharmacogenomics. 2000, 1: 309-333. 10.1517/14622416.1.3.309.
Segman RH, Cooper-Kazaz R, Macciardi F, Goltser T, Halfon Y, Dobroborski T, et al: Association between the dopamine transporter gene and posttraumatic stress disorder. Mol Psychiatry. 2002, 7: 903-907. 10.1038/sj.mp.4001085.
Bressan RA, Shih MC, Hoexter MQ, Lacerda AL: Can molecular imaging techniques identify biomarkers for neuropsychiatric disorders?. Rev Bras Psiquiatr. 2007, 29: 102-104.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB: Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995, 52: 1048-1060.
Wittchen HU, Lachner G, Wunderlich U, Pfister H: Test-retest reliability of the computerized DSM-IV version of the Munich-Composite International Diagnostic Interview (M-CIDI). Soc Psychiatry Psychiatr Epidemiol. 1998, 33: 568-578. 10.1007/s001270050095.
Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, et al: The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Arch Gen Psychiatry. 1992, 49: 630-636.
Spitzer RL, Williams JB, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992, 49: 624-629.
Del-Ben CM, Rodrigues CR, Zuardi AW: Reliability of the Portuguese version of the structured clinical interview for DSM-III-R (SCID) in a Brazilian sample of psychiatric outpatients. Braz J Med Biol Res. 1996, 29: 1675-1682.
Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al: The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995, 8: 75-90. 10.1002/jts.2490080106.
Beck AT, Epstein N, Brown G, Steer RA: An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988, 56: 893-897. 10.1037/0022-006X.56.6.893.
Gorenstein C, Andrade L, Vieira Filho AH, Tung TC, Artes R: Psychometric properties of the Portuguese version of the Beck Depression Inventory on Brazilian college students. J Clin Psychol. 1999, 55: 553-562. 10.1002/(SICI)1097-4679(199905)55:5<553::AID-JCLP3>3.0.CO;2-D.
Endicott J, Spitzer RL, Fleiss JL, Cohen J: The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976, 33: 766-771.
Weissman MM, Prusoff BA, Thompson WD, Harding PS, Myers JK: Social adjustment by self-report in a community sample and in psychiatric outpatients. J Nerv Ment Dis. 1978, 166: 317-326.
Weissman MM, Olfson M, Gameroff MJ, Feder A, Fuentes M: A comparison of three scales for assessing social functioning in primary care. Am J Psychiatry. 2001, 158: 460-466. 10.1176/appi.ajp.158.3.460.
Gorenstein C, Moreno RA, Bernik MA, Carvalho SC, Nicastri S, Cordas T, et al: Validation of the Portuguese version of the Social Adjustment Scale on Brazilian samples. J Affect Disord. 2002, 69: 167-175. 10.1016/S0165-0327(01)00300-7.
McHorney CA, Ware JE, Raczek AE: The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993, 31: 247-263. 10.1097/00005650-199303000-00006.
Severo M, Santos AC, Lopes C, Barros H: [Reliability and validity in measuring physical and mental health construct of the Portuguese version of MOS SF-36]. Acta Med Port. 2006, 19: 281-287.
Bremner JD, Bolus R, Mayer EA: Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007, 195: 211-218. 10.1097/01.nmd.0000243824.84651.6c.
Guy W: Patient assessment in clinical trials. Prog Neuropsychopharmacol Biol Psychiatry. 1982, 6: 601-606. 10.1016/S0278-5846(82)80155-3.
Marmar CR, Weiss D, Metzler T: The Peritraumatic Dissociative Experiences Questionnaire. Trauma, Memory, and Dissociation. Edited by: Bremner JD, Marmar CR. 1998, Wasshington, DC: American Psychiatric Press, 249-252.
Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G: Wisconsin Card Sorting Test Manual revised and expanded. 1993, Odessa: Psychological Assessment Recourses, Inc
Wechsler D: Wechsler adult intelligence scale, technical manual. 1997, San Antonio, TX: The Psychological Corporation, third
Spreen O, Strauss E: A Compendium of neuropsychological tests. 1998, New York: Oxford University Press, Second
Trenerry MR, Crosson B, DeBoe J, Leber WR: Stroop Neuropsychological Screening Test. 1989, Odessa, FL: Psychological Assessment Resources
Mesulan MM: Principles of Behavioral Neurology. 1985, Philadelphia: F.A.: Davis Company
Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, et al: Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci. 1992, 4: 125-133.
Talairach J, Tournoux P: Co-planar Stereotaxic Atlas of the Human Brain. 1988, New York: Thieme
Pantel J, O'Leary DS, Cretsinger K, Bockholt HJ, Keefe H, Magnotta VA, et al: A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus. 2000, 10: 752-758. 10.1002/1098-1063(2000)10:6<752::AID-HIPO1012>3.0.CO;2-Y.
Kung MP, Stevenson DA, Plossl K, Meegalla SK, Beckwith A, Essman WD, et al: [99mTc]TRODAT-1: a novel technetium-99m complex as a dopamine transporter imaging agent. Eur J Nucl Med. 1997, 24: 372-380.
Shih MC, Amaro E, Ferraz HB, Hoexter MQ, Goulart FO, Wagner J, et al: [Neuroimaging of the dopamine transporter in Parkinsons disease: first study using [99mTc]-TRODAT-1 and SPECT in Brazil]. Arq Neuropsiquiatr. 2006, 64: 628-634.
Snyder P, Nussbaum P: Clinical neuropsychology: a pocket handbook for assessment. 1998, Washington, DC: American Psychological Association
Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD: Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003, 54: 693-702. 10.1016/S0006-3223(03)00634-6.
Szobot CM, Shih MC, Schaefer T, Junior N, Hoexter MQ, Fu YK, et al: Methylphenidate DAT binding in adolescents with Attention-Deficit/Hyperactivity Disorder comorbid with Substance Use Disorder – a single photon emission computed tomography with [Tc(99m)]TRODAT-1 study. Neuroimage. 2008, 40: 1195-1201. 10.1016/j.neuroimage.2007.12.050.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-244X/9/30/prepub
Acknowledgements
This study was supported by the State of São Paulo Funding Agency (FAPESP) by the Grant: 2004/15039-0, and the National Research Council (CNPq) by the grant: 420122/2005-2. AFS had a master science grant from CNPq, and MCPC had a grant from the Ministry of Education (CAPES), 133485/2006-9). Prof. Jair Mari is a level I researcher from CNPq, under a sabbatical leave to the Health Services and Population Research Department, King's College, funded by The Brazilian Ministry of Education scholarship (CAPES). Ms. Denise Sessa was responsible for the administration of the grants. Wagner Ribeiro received a doctorate scholarship from CNPQ (141467/2007-0) and a one-year sandwich Capes scholarship (Proc.4516/07-9). Jair Barborsa Neto is under a M.Sc. CNPq scholarship and Dr. Karestan C. Koenen was supported by US-NIH grants K08-MH070627 and MH078928.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RAB, JJM, SBA, MFM, MRJ, WR, MIQ, IF have made a substantial contribution to the conception and design of the study and will be supervising data analysis and interpretation of data. ALTL and APJ participated in the structural neuroimaging planning of the project. RAB and MCS designed the molecular imaging section of the protocol. AFS, CA, JR, MH, and TSM are post-grad students involved in different parts of the project. CG and GB made a substantial contribution to the conception and design of the study, will be supervising data analysis and interpretation of data, particularly in the genoma analysis. DRL will be participating in the analysis and interpretation of data. JPF and LCQ are post-doc students and will be participating of data analysis and interpretation of the results. KCK will be supervising data analysis and interpretation of results. MCS did develop the SPECT study and is involved in data collection, analysis and interpretation of dopamine carriers. SM is a senior psychologist responsible for the neuropsychological assessments of the study (choice of instruments, training and accuracy of measurements).
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bressan, R.A., Quarantini, L.C., Andreoli, S.B. et al. The posttraumatic stress disorder project in Brazil: neuropsychological, structural and molecular neuroimaging studies in victims of urban violence. BMC Psychiatry 9, 30 (2009). https://doi.org/10.1186/1471-244X-9-30
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-244X-9-30