Abstract
Background
Women with bilateral abnormal uterine artery Doppler velocimetry (UtADV) are at increased risk for an adverse pregnancy outcome. This study aimed to determine if additional assessment of midtrimester angiogenic factors improves the predictive accuracy of Doppler results for various outcome parameters.
Methods
Women with a bilateral abnormal UtADV, which was defined as a postsystolic incision and/or an increased pulsatility index greater than the 95th centile, and a singleton pregnancy were prospectively recruited between 19 + 0 and 26 + 6 weeks of gestation. Maternal serum levels of placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFLT-1) were measured with a fully automated immunoassay and their ratio was calculated.
Results
Angiogenic factors could predict the development of preeclampsia (PE), as well as induced delivery at <34 weeks of gestation, but failed to predict the development of normotensive intrauterine growth restriction. Twelve (24.0%) of the 50 recruited women developed PE. Nine of these patients had early-onset disease (<34 + 0 weeks). Six (12.0%) patients were delivered at <34 + 0 weeks. The most useful test results in the prediction of PE and induced delivery at <34 + 0 weeks were observed using the sFLT-1/PlGF >95th centile ratio with a sensitivity, specificity, positive predictive value, and negative predictive value (NPV) of 66.7%, 89.5%, 66.7%, and 89.5% for PE, and 85.7%, 86.1%, 50.1%, and 97.4% for induced delivery, respectively. Positive and negative likelihood ratios were 6.33 (95% CI 2.31–17.38) and 0.37 (95% CI 0.17–0.84) for PE, and 6.14 (95% CI 2.76–13.69) and 0.17 (0.03–1.02) for induced delivery, respectively. Corresponding odds ratios were 17.0 (95% CI 3.5–83.0) and 37.0 (95% CI 3.8–363.9), respectively.
Conclusions
Measurement of angiogenic factors improves the specificity of an abnormal UtADV for prediction of PE. Compared with prediction of PE an abnormal sFLT-1/PlGF ratio revealed higher sensitivity for prediction of induced delivery at <34 + 0 weeks. The NPV of 97% will help to reassure most patients with an abnormal UtADV and a normal sFLT-1/PlGF ratio.
Similar content being viewed by others
Background
The estimated incidence of preeclampsia (PE) is between 0.4–2.8% of all pregnancies in Europe [1, 2]. Only 15–20% of PE is present a severe clinical course [3]. The majority of severe PE develops early in pregnancy (<34 weeks of gestation) and is frequently associated with a serious maternal and foetal outcome [4–6].
Angiogenic factors have a large effect on development of preeclamptic syndrome [7]. The extent of the antiangiogenic shift, characterized by an increase in antiangiogenic soluble fms-like tyrosine kinase (sFlt)-1 and a decrease in angiogenic placental growth factor (PlGF), correlates with disease severity and precedes the clinical manifestation for several weeks [8–11]. Therefore, analysis of maternal sFLT-1 and PlGF can improve the diagnostic accuracy for detection of PE and allows an estimation of the clinical disease severity [12]. Moreover, removal of sFLT-1 from the maternal circulation by apheresis improves the severity of the disease and consequently prolongs the duration of pregnancy [13].
Although definitive treatment of PE is possible by delivery of the placenta alone, early prediction of high-risk patients may enhance the patient´s care before disease manifestation and could help to reduce mortality and morbidity of the mother and her foetus. A widely used noninvasive approach for identifying high-risk patients comprises the performance of midtrimester Doppler ultrasound measurements of the uterine arteries. An abnormal uterine artery Doppler velocimetry (UtADV) during the second trimester has shown detection rates for PE between 40% and 80% in patients at low risk [14]. Generally, sensitivity is better for the detection of severe and/or early-onset PE compared to mild and late onset cases, but positive predictive accuracy is quite low, and the majority of patients with an abnormal UtADV will not develop PE [15]. However, additional analysis of angiogenic markers may improve the test accuracy for detecting PE. Our prospective study aimed to evaluate sFLT-1 and PlGF as predictive markers for PE in a high-risk collective as identified by an abnormal UtADV in the second trimester.
Methods
Selection and inclusion criteria of patients
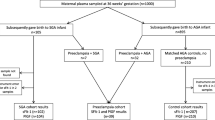
Patients with a bilateral abnormal UtADV between 19 + 0 and 26 + 6 weeks of gestation were included in this prospective cohort study. The clinical trial was conducted at the Department for Obstetrics and Gynaecology of the University of Rostock, Germany in cooperation with the outpatient centre of prenatal diagnosis “Praxiszentrum Frauenheilkunde” in Rostock between February 2011 and July 2013. The study was approved by the institutional review board of the University of Rostock (IRB No. A2010100) and written informed consent was obtained from all participating patients. An abnormal UtADV was assumed if a bilaterally increased pulsatility index (PI) greater than the 95th centile and/or a distinct postsystolic incision (“notch”) were detected [16]. All measurements were performed by two experienced observers following the recommendations for Doppler ultrasonography measurements in obstetrics [17]. For ultrasound examinations, the Voluson 730, Voluson G8 (both GE Medical Systems, Milwaukee, WI, USA), and HDI5000 SonoCT (Philips Medical Systems, Bothell, WA, USA) were used. Gestational age was calculated from the first day of the last menstrual period and was corrected by ultrasound if measurements of the crown-rump-length during the first trimester revealed a difference of more than 7 days. PE was defined as being present when blood pressure was ≥140/90 mmHg (taken twice, 6 h apart) combined with proteinuria ≥300 mg in a 24-h collection [18]. Inclusion of patients with chronic hypertension (blood pressure level ≥140/90 mmHg prior to 20 weeks of gestation) was possible. In these cases, new onset of proteinuria ≥300 mg per day was regarded as superimposed PE. According to the clinical manifestation, PE was assigned to early onset (<34 weeks of gestation) or late onset (≥34 weeks) [19]. Haemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome was defined as being present, when the platelet count was less than 100 × 109/L (normal range: 150–450 × 109/L), haptoglobin serum levels were below 0.3 g/L (normal range: 0.3–2.0 g/L), and aspartate transaminase (AST) levels were >70 U/L (normal range: 3–34 U/L). Only singleton pregnancies were included. Exclusion criteria were maternal age <18 years, on-going therapy with glucocorticoids or non-steroidal analgesics (with exception of aspirin 100 mg daily), and the presence of anti-phospholipid syndrome.
Small for gestational age (SGA) was defined as a birth weight less than the 10th centile for gestational age according to Voigt et al. [20]. Intrauterine growth restriction (IUGR) was assumed when a SGA birth weight was combined with at least one of the following criteria: (1) oligohydramnios with an amniotic fluid index <5 cm, (2) persistent bilateral abnormal UtADV, (3) a pathological cardiogram, (4) a decrease in foetal growth by ultrasound biometry (“crossing centiles”), and (5) increased PI of the umbilical artery greater than the 95th centile. Because clinicians were not aware of biomarker results, an effect of decision making for the obstetric approach was excluded.
Sample collection and immunoassays
From each study patient, 2 × 7.5 mL of venous blood was taken at the time of Doppler measurement using a serum collection tube (Sarstedt, Nümbrecht, Germany). The blood clot was immediately separated by centrifugation at 2000 × g for 10 min at 15°C and serum was stored in 1-mL aliquots at -80°C until further investigations were performed. Serum concentrations of PlGF and sFLT-1 were measured with the fully automated Elecsys® sandwich immunoassays (Roche Diagnostics, Penzberg, Germany) based on streptavidin-biotin technology. The assays were performed using the Roche immunoanalyser Elecsys® 2010/cobas e 411 according to the recommendations of the manufacturer. Within and between run coefficients of variation were below 4% for sFLT-1 > 60 pg/mL and PlGF >20 pg/mL. Functional sensitivity was <5 pg/mL [21, 22]. Results were classified using gestational age-dependent centile curves that were provided by the manufacturer and based on data from Verlohren and colleagues [10]. Results were regarded as pathological if sFLT-1 or the ratio of sFLT-1/PlGF was greater than the 95th centile and/or PlGF was less than the 5th centile.
Statistical analysis
All data were stored and analysed using the IBM SPSS statistical package 19.0 (SPSS Inc. Chicago, IL, USA) and Excel 2010 (Microsoft Corporation, Redmond, WA, USA). Descriptive statistics were computed for continuous and categorical variables. The statistics included mean, median, standard deviation (SD), minimum, maximum, and number of available observations of continuous variables. Values of continuous variables are presented as mean ± SD. For categorical variables, frequencies and relative frequencies are shown. Testing for differences of continuous variables between the groups was accomplished by the ANOVA or the Kruskal–Wallis test, as appropriate. Test selection was based on evaluation of the variables for normal distribution by using the Kolmogorov–Smirnov test. Comparison between the groups for categorical variables was performed using the chi-square test. All P values resulted from two-sided statistical tests and values of P < 0.05 were considered as statistically significant. Criteria of diagnostic validity for sFLT-1 and PlGF alone, as well as the sFLT-1/PlGF ratio, were computed and presented as sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio, and negative likelihood ratio. Furthermore, receiver operating characteristic (ROC) curves were calculated and the areas under the curves (AUCs) were reported. The most useful cut-off values were calculated by identification of the highest score for sensitivity and specificity. For the sFLT-1/PlGF ratio >95th centile, odds ratios (OR) were computed for various outcome parameters. The logistic regression model was used to assess the independence of specific outcome parameters. Variables yielding P values ≤0.05 in univariate analysis were entered in the multivariate model and adjusted for maternal body mass index, parity, onset of PE, existence of chronic hypertension, week of gestation at the moment of blood sampling, and use of acetylsalicylic acid. We performed post hoc analysis for estimation of the statistical power of our study collective by logistic regression analysis using G*Power 3.1.9.2 [23]. One-tailed power analysis for prediction of PE by the sFLT-1/PlGF ratio with an α error of 0.05 revealed a statistical power of 95.5% for our sample size.
Results
Patients’ characteristics
The patients’ characteristics are shown in Table 1 with the following prevalence of subgroups: PE was found in 24.0% (12/50) of patients, IUGR in 12.0% (6/50), gestational hypertension in 32.0% (16/50), and others in 32.0% (16/50). Subgroup analysis did not show any differences between maternal characteristics, such as age, gravidity, parity, and body mass index. In contrast, significant intergroup differences were found regarding foetal/neonatal parameters. More than 40% of all preeclamptic newborns showed a SGA birth weight. Newborns in the PE subgroup also presented with the lowest mean gestational age. Consequently, the mean birth weight in cases of PE was significantly lower compared with that in the other subgroups, including newborns with normotensive IUGR. Delivery at <34 + 0 weeks occurred in 14.0% (7/50) of all patients. Five of them (71.4%) had PE and two had severe hypertension without proteinuria. All of these patients had an induced delivery because of a severe clinical course indicated by the physicians on duty. One patient with chronic hypertension and concomitant diabetes mellitus type II had an intrauterine foetal death at 26 weeks of gestation. Induction of lung maturation with betamethasone was not performed in three newborns with induced delivery at <34 + 0 weeks, including the patient with intrauterine foetal death. Doppler measurements were repeated in all of the patients after 4 weeks. An abnormal UtADV normalized in 32.0% (16/50) of all cases. There were significant differences between subgroups (P = 0.047) with normalization of an abnormal uterine Doppler in 0.0% of PE (0/12), 33.3% of IUGR (2/6), 31.3% of gestational hypertension (5/16), and 56.3% in the remaining patients (9/16).
Prediction of PE by UtADV, PlGF, sFLT-1, and the sFLT-1/PlGF ratio
The prevalence of PE in our high-risk study collective was 24.0% (12/50), with 75.0% (9/12) early-onset and 25.0% (3/12) late-onset diseases. PE was also present with chronic hypertension in four cases (all early onset). One patient developed concomitant HELLP syndrome. For prediction of PE, bilateral UtADV showed a PPV of only 24.0% with a false-positive rate (FPR) of 76.0%. Performance of ROC analysis using the mean PI of the uterine arteries showed an AUC of 0.630. At the optimal cut-off with a mean uterine PI ≥1.86, the specificity increased to 72.2%, with a PPV of 38.2%. However, the false negative rate also increased to 45.5%, resulting in a predictive accuracy of only 70.0%. In contrast, serum levels of PlGF and sFLT-1, as well as the sFLT-1/PlGF ratio, clearly improved discrimination between patients with and without PE (Figure 1, Table 2). If values were greater than the 95th centile of gestation, the sFLT-1/PlGF ratio showed most useful test characteristics for PE, with a sensitivity of 66.7% and an FPR of only 10.5% (Table 3). The corresponding PPV was 66.7%, with an NPV of 89.5% and a predictive accuracy of the ratio of 84.0%. An elevated sFLT-1/PlGF ratio greater than the 95th centile was associated with an increased risk for development of PE (OR 17.0; 95% confidence interval [CI] 3.5–83.0, P < 0.001) (Table 3). Results remained significant after weighting of parameters in multiple regression analysis with an adjusted OR for PE (adjusted OR 14.1; 95% CI 1.1–182.6, P = 0.043).
Prediction of PE. Boxplot analysis of maternal serum PlGF (A) and sFLT-1 (B) levels and their ratio (C). Patients with development of preeclampsia (PE, n = 12) were compared with all patients without PE (n = 38). Boxes show the median (black line), and 25th, and 75th percentiles (top and bottom lines of the box). The lines outside the box represent minimum and maximum values. Circles are outliers (>1.5× from the interquartile range). P values were computed by Mann–Whitney statistical tests.
The sensitivity increased to 75.0% if only early-onset PE was included, but FPR also increased to 14.3%. We found four false-positive cases, where one patient showed a normotensive IUGR and two had hypertension without proteinuria (one with concomitant IUGR and one with intrauterine foetal death at 26 weeks of gestation). Two out of the four false-negative patients developed early-onset PE (one patient with concomitant HELLP syndrome at <34 weeks of gestation) and two developed late-onset PE (with only a mild clinical course in one patient). The fourth patient with false-negative serum markers developed late-onset PE, but fulfilled the criteria of severity with hypertension >160/110 mmHg. ROC analysis showed an AUC of 0.721 for the sFLT-1/PlGF ratio, with the best predictive cut-off of 13.0, resulting in a sensitivity of 66.7% with an FPR of 13.2% (Figure 2, Table 4). If a fixed FPR of 10% was assumed, the cut-off level of the ratio changed to 22.4, with a sensitivity of only 41.7%.
Prediction of hypertension without proteinuria, IUGR, and induced delivery at <34 weeks of gestation
Results were further analysed in respect to development of hypertension without proteinuria and IUGR. As shown in Table 2, serum levels of PlGF were significantly lower in cases of IUGR compared to uncomplicated controls (P = 0.033), but failed significance if used as predictive parameter throughout the whole study population (Table 3). However, we found significant differences of the angiogenic markers if results were analysed in relation to gestational age at delivery (Figure 3). Measurements of sFLT-1 and PlGF were useful predictive markers for delivery at <34 + 0 weeks of gestation. If ROC analysis was performed, the AUC for the sFLT-1/PlGF ratio was 0.877, with a best cut-off of 13.0 (Figure 2). This cut-off resulted in a sensitivity of 85.7% with an FPR of 16.3%. If gestational age-dependent threshold levels greater than the 95th centile were used, the FPR improved to 13.9% with the same sensitivity. The PPV and NPV were 50.1% and 97.4%, respectively. The ratio of sFLT-1/PlGF showed a predictive accuracy of 86.0%. An elevated sFLT-1/PlGF ratio greater than the 95th centile was associated with an increased risk for delivery at <34 + 0 weeks (OR 37.0; 95% CI 3.8–363.9, P < 0.001) (Table 3). Results remained significant after weighting of parameters in multiple regression analysis with an adjusted OR (34.6; 95% CI 1.2–1026.2, P = 0.041).
Prediction of preterm delivery < 34 weeks. Boxplot analysis of maternal serum PlGF (A) and sFLT-1 (B) levels and their ratio (C). Patients with delivery at <34 weeks of gestation (n = 6) were compared with the remaining patients (n = 44). Boxes show the median (black line), and 25th and 75th percentiles (top and bottom lines of the box). The lines outside the box represent minimum and maximum values. Circles are outliers (>1.5× from the interquartile range). P values were computed by Mann–Whitney statistical tests.
Discussion
Main findings
Patients with an abnormal UtADV showed distinct foetal and maternal morbidity, with a high prevalence of PE, IUGR, and hypertensive disorders without proteinuria. However, only one of four patients with abnormal UtADV developed PE. Additional measurement of the sFLT-1/PlGF ratio was able to decrease the FPR from 76.0% to 10.5%. A negative test result below the 95th gestational age-specific centile showed an NPV of 89.5%. The NPV was further improved if only patients with early onset PE (94.7%) or those who required delivery at <34 weeks (97.4%) were considered. Therefore, women with a normal sFLT-1/PlGF ratio were at low risk for development of one of these severe clinical courses. Additional measurement of sFLT-1 and PlGF would be helpful to reassure the majority of patients with an abnormal UtADV. An increased angiogenic ratio was associated with an increased risk of a severe hypertensive disorder and concomitant preterm delivery. Provident corticosteroid treatment of these women should be considered.
Interpretation
There are different approaches for prediction of PE. Doppler ultrasound of the uterine arteries during the second trimester is the best-known single predictor for PE, with a detection rate up to 80% for early-onset disease [14, 24–26]. However, the majority of patients with abnormal UtADV will never develop PE, which suggests the need to improve the predictive accuracy. Therefore, use of a well-established and widely performed test for identification of a high risk collective and its combination with measurement of angiogenic factors for improvement of specificity is a practically oriented approach. The use of a fully automated diagnostic system that analyses sFLT-1 and PlGF guarantees a high test quality. The Elecsys® immunoassays show high precision and reliability, and allow execution of measurements in laboratories for routine clinical diagnosis [21].
Recent studies have reported complex algorithms that allow prediction of PE by integration of miscellaneous risk factors, including analysis of angiogenic factors between 11–13 weeks of gestation [27–29]. Identification of high-risk patients during the first trimester offers the advantage of a preventive therapy with low-dose aspirin, which more than halves the risk of severe PE [30]. However, the ability of prediction or exclusion of PE during the second trimester may also be useful because it can facilitate decision making as to whether intensified patient’s care or corticosteroid treatment for foetal lung maturation will be necessary. There is also increasing evidence for effective preventive treatment strategies in high-risk patients during the second trimester [31–33]. Various (nested) case–control studies have examined the differences in maternal angiogenic protein concentrations during the second trimester preceding the occurrence of PE [8, 34–41]. The usefulness of circulating sFLT-1 and PlGF as a test to predict PE in healthy pregnant women was recently proven in a comprehensive prospective cohort study by McElrath and colleagues [11]. In a cohort of 2243 patients, the plasma levels of angiogenic factors were repeatedly measured at 10, 17, 24, and 35 weeks of gestation. In the study sample, 139 (6.2%) patients developed PE. At 10 weeks of gestation, the authors did not find any significant differences between plasma concentrations of PlGF or sFLT-1, or the sFLT-1/PIGF ratio in patients with PE compared with healthy controls. Differences became significant at 17 weeks for PlGF (P = 0.009) alone, and were highly significant for PlGF and the sFLT-1/PIGF ratio at 24 weeks (P < 0.001). In contrast, differences for sFLT-1 became significant at 35 weeks. At 24 weeks, the highest detection rate (62.2%) was observed for PlGF alone, with an FPR of 38.4% and a PPV of 9.5%. The relative risk for development of PE was 2.46 (95% CI 1.66–-3.65) if a cut-off level of 379 pg/mL was used [11]. Similar results were shown in a further prospective study including 1622 healthy Hispanic women [25]. Maternal plasma concentrations of various angiogenic proteins were measured at 6–15 and 20–25 weeks of gestation. During pregnancy, 3.8% of patients developed PE (n = 62), and 0.6% had early-onset PE (n = 9). In midtrimester, PlGF measurements alone showed a better test performance (P < 0.001) than sFLT-1 (P = 0.5) or the sFLT-1/PIGF ratio (P = 0.006). However, the detection rate for PlGF was only 51.6%, with an FPR of 23.6% and a PPV of 8%. A reasonable cut-off value was 215 pg/mL, resulting in an OR of 3.8 (95% CI 2.2–6.7). The detection rate for early-onset PE was 100%, and was therefore better, with an FPR of only 4.2% at a cut-off level of 126 pg/mL [25]. The authors of both studies concluded that angiogenic protein concentrations alone, measured in early or even late pregnancy, were of limited utility for prediction of PE. For prediction of PE, its low incidence and concomitant low predictive value are problematic. Therefore, identification of high-risk patients may be an alternative, more suitable approach. In a prospective cohort study of 3348 patients, Espinoza and colleagues examined the relationship between UtADV and plasma concentrations of PlGF and sFLT-1 between 22 and 26 weeks of gestation [24]. In this study, they showed that abnormal UtADV and maternal PlGF were independent explanatory variables for the occurrence of PE. For prediction of PE, the FPR of abnormal UtADV alone was low (10.4%). Abnormal UtADV had a better test performance than maternal PlGF for identification of a high-risk collective, with ORs for all types of PE and early-onset PE of 4.3 (95% CI 2.82–6.66) and 24.1 (95% CI 9.61–60.44) compared with PlGF (cut-off, 280 pg/mL), with corresponding ORs of 2.6 (95% CI 1.57–3.94) and 5.5 (95% CI 1.98–15.08). The combination of both parameters increased the OR for early-onset PE to 43.8 (95% CI 18.48–103.9). The PPV for early-onset PE was improved from 5.4% for abnormal UtADV and 1.4% for PlGF alone to 12.7% if both factors were combined with a positive likelihood ratio of 18.48 (95% CI 13.07–26.13). This study [24] impressively showed that PlGF significantly contributed to the prediction of patients who were going to develop early-onset PE, and thus confirmed the results of a smaller study by Stepan and colleagues [42]. The supplemental table compares the test characteristics of the sFLT-1/PlGF ratio of various studies (Additional file 1: Table S1). The prospective cohort study by Moore Simas and colleagues included only patients with at least one risk factor for PE in their medical history [43]. In this study, the determination of sFLT-1 showed a good predictive accuracy for early-onset PE, with an AUC value of 0.90 (95% CI 0.78–1.0) between 22 and 26 weeks of gestation. The sFLT-1/PlGF ratio was even better with an AUC value of 0.97 (95% CI 0.91–1.0). Similar results of other studies, including our own data, showed an increase in specificity if the sFLT-1/PlGF ratio instead of PlGF alone was used for prediction of early-onset PE. However, compared with a decrease in PlGF, an increase in sFLT-1 in patients with PE is a late event, with only a narrow time frame between diagnosis and clinical manifestation of PE [8, 11].
Although the end-point of our study was the development of PE, we additionally analysed the correlation between angiogenic factors and the occurrence of other adverse outcome parameters, such as IUGR and delivery at <34 weeks. The group of induced deliveries showed great overlap with the PE cases and could be regarded as a surrogate for a severe clinical course. Therefore, identification of these patients is of high clinical relevance. With an AUC of 0.88, we observed a satisfactory test performance for prediction of cases with a delivery at <34 weeks of gestation. Although PPV was only 50.1%, we found an NPV of 97.4%. In patients with abnormal UtADV and a normal angiogenic ratio, the need for delivery at <34 weeks following hypertensive pregnancy disorders is highly unlikely. With a sensitivity of 71% and a specificity of 76%, Stepan and colleagues also reported the capability of the sFLT-1/PlGF ratio for prediction of preterm delivery [42]. Additionally, in a prospective study of patients with suspected early-onset PE, the sFLT-1/PlGF ratio showed prognostic relevance [44]. Patients with a ratio ≥85 had a significantly increased risk for delivery within the next 2 weeks, with a hazard ratio of 15.2 (95% CI 8.0–28.7).
In our study, angiogenic factors were not suitable for prediction of hypertension without proteinuria or for normotensive IUGR, although in IUGR, the levels of PlGF were significantly lower compared with inconspicuous controls. Differences reported in other studies were also only moderate, and not helpful for diagnosis or prediction of these adverse pregnancy outcome parameters [12, 41, 42].
Conclusion
In high-risk patients, measurement of angiogenic factors shows a good predictive power for development of PE. The best results can be achieved using the ratio of sFLT-1 and PlGF, with a predictive test accuracy of 84%. In patients with abnormal UtADV, a high FPR of Doppler measurement alone is improved to 10.5%. An increased sFLT-1/PlGF ratio is also predictive for patients who need delivery at <34 + 0 weeks of gestation. The test performed even better for prediction of preterm delivery compared with PE, with a sensitivity and specificity of >80%. In particular, a NPV of 97.4% can help to reassure most patients with an abnormal UtADV.
References
Dolea C, AbouZahr C: Global burden of hypertensive disorders of pregnancy in the year 2000. Global Burden of Disease 2000. 2003, Geneva: World Health Organization, http://www.who.int/healthinfo/statistics/bod_hypertensivedisordersofpregnancy.pdf,
Gaillard R, Arends LR, Steegers EA, Hofman A, Jaddoe VW: Second- and third-trimester placental hemodynamics and the risks of pregnancy complications: the Generation R Study. Am J Epidemiol. 2013, 177: 743-754.
Zhang WH, Alexander S, Bouvier-Colle MH, Macfarlane A: Incidence of severe pre-eclampsia, postpartum haemorrhage and sepsis as a surrogate marker for severe maternal morbidity in a European population-based study: the MOMS-B survey. BJOG. 2005, 112: 89-96.
Lisonkova S, Joseph KS: Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013, 209: 544-
MacKay AP, Berg CJ, Atrash HK: Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001, 97: 533-538.
Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R: Preeclampsia and fetal growth. Obstet Gynecol. 2000, 96: 950-955.
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003, 111: 649-658.
Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA: Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004, 350: 672-683.
Reimer T, Rohrmann H, Stubert J, Pecks U, Glocker MO, Richter DU, Gerber B: Angiogenic factors and acute-phase proteins in serum samples of preeclampsia and HELLP patients: a matched-pair analysis. J Matern Fetal Neonatal Med. 2013, 26: 263-269.
Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, Pape J, Dudenhausen JW, Denk B, Stepan H: An automated method for the determination of the sFLT-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010, 202: 161-
McElrath TF, Lim KH, Pare E, Rich-Edwards J, Pucci D, Troisi R, Parry S: Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol. 2012, 207: 407-
McElrath TF, Lim KH, Pare E, Rich-Edwards J, Pucci D, Troisi R, Parry S: The sFLT-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012, 206: 58-
Thadhani R, Kisner T, Hagmann H, Bossung V, Noack S, Schaarschmidt W, Jank A, Kribs A, Cornely OA, Kreyssig C, Hemphill L, Rigby AC, Khedkar S, Lindner TH, Mallmann P, Stepan H, Karumanchi SA, Benzing T: Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011, 124: 940-950.
Cnossen JS, Morris RK, ter Riet G, Mol BW, van der Post JA, Coomarasamy A, Zwinderman AH, Robson SC, Bindels PJ, Kleijnen J, Khan KS: Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ. 2008, 178: 701-711.
Myatt L, Clifton RG, Roberts JM, Spong CY, Hauth JC, Varner MW, Wapner RJ, Thorp JM, Mercer BM, Grobman WA, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Tolosa JE, Saade G, Sorokin Y, Anderson GD: The utility of uterine artery Doppler velocimetry in prediction of preeclampsia in a low-risk population. Obstet Gynecol. 2012, 120: 815-822.
Schaffer H: Doppler-Referenzkurven. Dopplersonographie in Geburtshilfe und Gynäkologie. 2000, Heidelberg Berlin New York: Springer Verlag, 292-293.
Bhide A, Acharya G, Bilardo CM, Brezinka C, Cafici D, Hernandez-Andrade E, Kalache K, Kingdom J, Kiserud T, Lee W, Lees C, Leung KY, Malinger G, Mari G, Prefumo F, Sepulveda W, Trudinger B: ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol. 2013, 41: 233-239.
Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000, 183: S1-S22.
von Dadelszen P, Magee LA, Roberts JM: Subclassification of preeclampsia. Hypertens Pregnancy. 2003, 22: 143-148.
Voigt M, Rochow N, Hesse V, Olbertz D, Schneider KT, Jorch G: Short communication about percentile values of body measures of newborn babies. Z Geburtshilfe Neonatol. 2010, 214: 24-29.
Schiettecatte J, Russcher H, Anckaert E, Mees M, Leeser B, Tirelli AS, Fiedler GM, Luthe H, Denk B, Smitz J: Multicenter evaluation of the first automated Elecsys sFLT-1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem. 2010, 43: 768-770.
Ohkuchi A, Hirashima C, Suzuki H, Takahashi K, Yoshida M, Matsubara S, Suzuki M: Evaluation of a new and automated electrochemiluminescence immunoassay for plasma sFLT-1 and PlGF levels in women with preeclampsia. Hypertens Res. 2010, 33: 422-427.
Faul F, Erdfelder E, Buchner A, Lang AG: Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009, 41: 1149-1160.
Espinoza J, Kusanovic JP, Bahado-Singh R, Gervasi MT, Romero R, Lee W, Vaisbuch E, Mazaki-Tovi S, Mittal P, Gotsch F, Erez O, Gomez R, Yeo L, Hassan SS: Should bilateral uterine artery notching be used in the risk assessment for preeclampsia, small-for-gestational-age, and gestational hypertension?. J Ultrasound Med. 2010, 29: 1103-1115.
Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Edwin SS, Gomez R, Yeo L, Conde-Agudelo A, Hassan SS: A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009, 22: 1021-1038.
Velauthar L, Plana MN, Kalidindi M, Zamora J, Thilaganathan B, Illanes SE, Khan KS, Aquilina J, Thangaratinam S: Uterine artery Doppler in the first trimester as a risk factor for adverse pregnancy outcomes: A meta-analysis involving 55,974 women. Ultrasound Obstet Gynecol. 2013, 43: 500-507.
Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH: Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013, 33: 8-15.
Myers JE, Kenny LC, McCowan LM, Chan EH, Dekker GA, Poston L, Simpson NA, North RA: Angiogenic factors combined with clinical risk factors to predict preterm pre-eclampsia in nulliparous women: a predictive test accuracy study. BJOG. 2013, 120: 1215-1223.
Scazzocchio E, Figueras F, Crispi F, Meler E, Masoller N, Mula R, Gratacos E: Performance of a first-trimester screening of preeclampsia in a routine care low-risk setting. Am J Obstet Gynecol. 2013, 208: 203-
Roberge S, Giguere Y, Villa P, Nicolaides K, Vainio M, Forest JC, von Dadelszen P, Vaiman D, Tapp S, Bujold E: Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: a systematic review and meta-analysis. Am J Perinatol. 2013, 29: 551-556.
Schleussner E, Lehmann T, Kahler C, Schneider U, Schlembach D, Groten T: Impact of the nitric oxide-donor pentaerythrityl-tetranitrate on perinatal outcome in risk pregnancies: a prospective, randomized, double-blinded trial. J Perinat Med. 2014, 42: 507-514.
Becker R, Keller T, Kiesewetter H, Fangerau H, Bittner U: Individual risk assessment of adverse pregnancy outcome by multivariate regression analysis may serve as basis for drug intervention studies: retrospective analysis of 426 high-risk patients including ethical aspects. Arch Gynecol Obstet. 2013, 288: 41-48.
Belizan JM, Villar J, Gonzalez L, Campodonico L, Bergel E: Calcium supplementation to prevent hypertensive disorders of pregnancy. N Engl J Med. 1991, 325: 1399-1405.
Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS: Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001, 184: 1267-1272.
Villa PM, Hamalainen E, Maki A, Raikkonen K, Pesonen AK, Taipale P, Kajantie E, Laivuori H: Vasoactive agents for the prediction of early- and late-onset preeclampsia in a high-risk cohort. BMC Pregnancy Childbirth. 2013, 13: 110-
Krauss T, Pauer HU, Augustin HG: Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens Pregnancy. 2004, 23: 101-111.
Hassan MF, Rund NM, Salama AH: An Elevated Maternal Plasma Soluble fms-Like Tyrosine Kinase-1 to Placental Growth Factor Ratio at Midtrimester Is a Useful Predictor for Preeclampsia. Obstet Gynecol Int. 2013, 2013: 202346-
Unal ER, Robinson CJ, Johnson DD, Chang EY: Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. Am J Obstet Gynecol. 2007, 197: 211-214.
Tjoa ML, van Vugt JM, Mulders MA, Schutgens RB, Oudejans CB, van Wijk IJ: Plasma placenta growth factor levels in midtrimester pregnancies. Obstet Gynecol. 2001, 98: 600-607.
Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC: Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol. 2007, 196: 239-6.
Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA: A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008, 21: 9-23.
Stepan H, Unversucht A, Wessel N, Faber R: Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension. 2007, 49: 818-824.
Moore Simas TA, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE: Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol. 2007, 197: 244-248.
Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA: Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012, 125: 911-919.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2393/14/292/prepub
Acknowledgements
The authors would like to thank Ms Erika Greschkowitz for her assistance in laboratory processing. The study was financed by own resources of the department of Obstetrics and Gynaecology of the University of Rostock.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JS conceived and designed the study, analysed and interpreted the data, and drafted the manuscript. SU acquired and analysed the patients’ data, and drafted the manuscript. MB acquired patients, performed the ultrasound examination and took blood samples. TK acquired patients, performed the ultrasound examination and took blood samples. MD analysed and interpreted the data, and wrote the manuscript. DUR designed the study and acquired the lab data. TR conceived and designed the study, and wrote the manuscript. All authors have seen and approved the final version of the manuscript.
Electronic supplementary material
12884_2014_1174_MOESM1_ESM.doc
Additional file 1: Table S1: Test indices of the midtrimester sFlt-1/PlGF ratio for prediction of preeclampsia. Comparison of relevant studies (see Discussion). (DOC 37 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Stubert, J., Ullmann, S., Bolz, M. et al. Prediction of preeclampsia and induced delivery at <34 weeks gestation by sFLT-1 and PlGF in patients with abnormal midtrimester uterine Doppler velocimetry: a prospective cohort analysis. BMC Pregnancy Childbirth 14, 292 (2014). https://doi.org/10.1186/1471-2393-14-292
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2393-14-292