Abstract
Background
The national incidence of and risk factors for hospitalized poisonings in renal transplant recipients has not been reported.
Methods
Historical cohort study of 39,628 renal transplant recipients in the United States Renal Data System between 1 July 1994 and 30 June 1998. Associations with time to hospitalizations for a primary diagnosis of poisonings (ICD-9 codes 960.x-989.x) within three years after renal transplant were assessed by Cox Regression.
Results
The incidence of hospitalized poisonings was 2.3 patients per 1000 person years. The most frequent causes of poisonings were immunosuppressive agents (25.3%), analgesics/antipyretics (14.1%), psychotropic agents (10.0%), and insulin/antidiabetic agents (7.1%). In Cox Regression analysis, low body mass index (BMI, <21.6 vs. >28.3 kg/m2, adjusted hazard ratio (AHR), 3.02, 95% CI, 1.45–6.28, and allograft rejection, AHR 1.83, 95% CI, 1.15–2.89, were the only factors independently associated with hospitalized poisonings. Hospitalized poisonings were independently associated with increased mortality (AHR, 1.54, 95% CI 1.22–1.92, p = 0.002).
Conclusions
Hospitalized poisonings were associated with increased mortality after renal transplantation. However, almost all reported poisonings in renal transplant recipients were due to the use of prescribed medications. Allograft rejection and low BMI were the only independent risk factors for poisonings identified in this population.
Similar content being viewed by others
Background
Poisonings are among the most frequent indications for hospitalization in the United States [1]. However, the frequency of hospitalizations for poisonings (drug overdose or toxicity) in renal transplant recipients has been infrequently reported, [2, 3] in contrast to its occurrence in kidney donors [4, 5]. Because many medications used by transplant recipients can be toxic, it might be expected that renal transplant recipients would have a higher risk of poisonings than the general population, especially in the first post-transplant year when dosages of immunosuppressive medications are usually at their highest. Analysis of the incidence and causes of poisonings represents an opportunity to explore the frequency and risk factors for medical errors, since many such complications may be avoidable. The impact of hospitalized poisonings on subsequent survival after renal transplantation has also not been assessed. Therefore, we analyzed national diata from the USRDS report. Our objectives were to determine the risk factors for and mortality associated with hospitalized poisonings after renal transplantation.
Methods
Patient population
This study used data from the United States Renal Data System (USRDS), using standard analysis files (SAF's) as of May 2000. The variables included in the USRDS standard analysis files (SAF's), as well as data collection methods and validation studies, are listed at the USRDS website, under 'Researcher's Guide to the USRDS Database', Section E, 'Contents of all the SAF's', http://www.usrds.org and published in the USRDS. The demographics of the renal transplant population have been previously described (2001 USRDS report). SAF.TXUNOS was used as the primary dataset, and merged with variables from SAF.HOSP for hospitalization data, and SAF.PATIENTS for dates and causes of death as well as causes of renal disease, as previously reported [6–8]. Patient characteristics and treatment factors were those at the date of transplant. Recipients of organs other than kidneys were excluded.
Outcome definition
We conducted an historical cohort study of the incidence, risk factors and associated patient survival for hospitalized cases of poisonings (based on International Classification of Diseases-9th Modification Diagnosis Codes (ICD9) 960.x-989.x) as a primary discharge diagnosis in renal transplant recipients. Only the primary discharge diagnosis was used to ensure these were active diagnoses, ie, to exclude diagnoses with "history of poisonings." These diagnoses include potential overdoses for heroin, but not for cocaine or other illicit drugs. These diagnoses also include most known causes of environmental exposures, including minerals, pesticides, vaccinations, miscellaneous chemicals and certain foodstuffs. The first hospitalization for poisonings after the first renal transplant for a given individual occurring on or after 1 July 1994 and before 1 July 1998 (which could include a repeat transplant), with followup time truncated at three years was counted in analysis. Hospitalizations were chosen because they were more accessible in the database and less subject to interpretation than outpatient cases of poisonings, especially since the USRDS database has no information on confirmatory studies. Hospitalization data for transplant recipients may be unreliable after the patient has survived ≥ 3 years post transplant, when hospitalization reporting to Medicare for patients 65 years or younger is no longer required. However, Medicare reporting starts immediately after transplant, regardless of preceding dialysis status. All hospitalizations with a primary discharge diagnosis for poisonings were extracted from SAF.HOSP, merged with the transplant file, and hospitalizations outside the range of the study period were excluded. Hospitalizations for poisonings occurring at any time after renal transplant, including after graft failure (censored for patient death), were counted in analysis.
Variables used in analysis
The independent associations between patient factors and hospitalizations for poisonings were examined using multivariate analysis with stepwise Cox Regression (likelihood ratio method) including recipient and donor age, recipient race, gender, weight, pretransplant dialysis (yes/no), year of transplant, duration of dialysis prior to transplantation, total follow-up time, repeat transplant, donor cytomegalovirus serology, dialysis in the first week after transplant (delayed graft function, yes/no), rejection (diagnosis or treatment) occurring during the first six months after transplant, induction antibody therapy, maintenance immunosuppressive medications at time of discharge after transplant surgery, graft loss (analyzed as a time-dependent covariate as previously described), [9] and cause of ESRD (diabetes, systemic lupus erythematosus (because of the potential effect of corticosteroid therapy prior to renal transplantation), hypertension and glomerulonephritis). Episodes of rejection were not restricted to those occurring in the first year, in contrast to studies of allograft function, since there is no evidence that late (vs. early) rejection has a different impact on poisonings. The total cumulative dose of prednisone was not available in the USRDS. Both the USRDS and UNOS track the numbers of days of prednisone administered prior to initial hospital discharge, however values were missing for >90% of patients in both databases and could not be used as a covariate in the above analyses. Maintenance immunosuppressive medication use at the time of discharge after transplantation was also analyzed as a preexisting covariate. Information on use of medications (other than immunosuppressive medications), alcohol, tobacco, or radiologic procedures was not available.
Survival times
We defined patient survival times as the time from when patients had their first renal transplant during the study period to the time of death or most recent follow-up date (which was considered May 2000). For time to poisonings, survival time was defined as the time from first renal transplant until hospitalization for poisonings, with patients censored at death, loss to followup, or end of the study (which was considered 31 December 1999, since this was the most recent hospitalization date). The patient survival probabilities were estimated by using the Kaplan Meier method.
Statistical analysis
All analyses were performed using SPSS 9.0 TM (SPSS, Inc., Chicago, IL). Files were merged and converted to SPSS files using DBMS/Copy (Conceptual Software, Houston, TX). Univariate analysis was performed with Chi-square testing for categorical variables and Student's two-sided t-test for continuous variables. Variables with p < 0.05 in univariate analysis for a relationship with development of hospitalization for poisonings were entered into multivariate analysis as covariates. Kaplan-Meier analysis was used to construct survival plots of time to hospitalized poisonings after renal transplantation. Log-log plots were inspected to assess for proportionality of hazards over time. Stepwise Cox proportional hazards regression (likelihood ratio method) was used to model the association of patient factors with hospitalized poisonings. In order to control for misspecification errors, a random sample of 25% of patients was excluded from analysis and coefficients estimated for this sample. The sample was frozen, and coefficients estimated from the 25% sample. Because hospitalizations for poisonings were uncommon, Poisson Regression was used for validation, using the GENLOG approximation in SPSS. In order to estimate the association of hospitalizations with all-cause mortality, Cox-nonproportional hazards Regression was utilized. Times after hospitalization for poisonings were entered as 1, all other times were entered as 0, as previously described [10, 11]. Patients with missing information for variables were excluded from the multivariate models, resulting in models smaller than the total population. Hierarchically well-formed models were used for the assessment of interaction terms [11].
Results
There were 42,096 solitary renal transplant recipients in the United States Renal Data System transplanted from 1 July 1994 to 30 June 1998, of whom 39,628 had complete enough information to calculate survival times. Mean followup was 1.89 ± 1.15 years (median, 1.91 years). Of the study population, 170 recipients were hospitalized with a primary discharge diagnosis of poisonings during the study period, with 188 total hospitalizations. The cumulative incidence of hospitalizations for poisonings during the study period was 2.3/1000 person years. Primary diagnoses for hospitalized poisonings are shown in Table 1. Suicide was not listed as an eventual primary cause of death among patients hospitalized for poisonings, nor was attempted suicide (E956, "Suicide and self-inflicted injuries by cutting and piercing instrument," or E958.9 "unspecified cause of suicide") listed as a secondary or tertiary discharge diagnosis among these patients. The mean length of stay for patients hospitalized for poisonings was 4.05 ± 4.15 days (range, 1–41 days). Among renal transplant recipients with diabetes, the most common cause of hospitalized poisonings was insulin/antidiabetic agents, while in patients who experienced allograft rejection, the most common cause of hospitalized poisonings was immunosuppressive agents.
The time to hospitalization for poisonings is shown in Figure 1. As shown, the risk of hospitalized poisonings were highest in the first few months after renal transplantation, with a consistent risk afterward. Figures 2 and 3 show the time to hospitalization for poisoning stratified by allograft rejection and body mass index, respectively. In Figure 2, analysis was limited to patients who survived at least 6 months after transplant. In figure 3, the risk of poisonings for all transplant recipients was similar for the first six months by both survival plots and log-log plots. Therefore, since this violated the proportional hazards assumption, multivariate analysis was also performed limited to patients who survived at least six months after transplant.
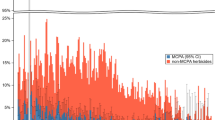
Time to Hospitalization for Poisonings after Renal Transplantation, stratified by allograft rejection occurring within 6 months after transplant. Patients with a history of allograft rejection (REJ) had a significantly higher risk of hospitalized poisoning than those who did not (p = 0.01 by Log Rank Test).
Time to Hospitalization for Poisonings after Renal Transplantation, stratified by quartiles of BMI (1 =< 21.6 kg/m2, 2 = 21.6–24.9 kg/m2, 3 = 25.0–28.3 kg/m2, 4 => 28.3 kg/m2). Recipients with BMI < 21.6 kg/m2 had a significantly higher risk of hospitalized poisonings compared to recipients with BMI > 28.3 kg/m2, p = 0.01 by Log Rank Test.
Characteristics of the study population, including results of both univariate and multivariate analysis, are shown in table 2 (see additional file 1). In univariate analysis, factors associated with an increased risk of hospitalized poisonings were low body mass index, graft loss, cadaveric donor, rejection within 6 months after transplant, and diabetes, while male gender was associated with reduced risk of poisoning. In Cox Regression analysis, however, the only two factors independently associated with hospitalized poisoning were low body mass index and allograft rejection. There were no significant interactions between covariates, specifically no interactions between maintenance or induction medications, or between age and body mass index. In analysis limited to recipients age 18 and over, low body mass index (as assessed in Table 3, AHR, 2.74, 95% CI, 1.29–5.82, p = 0.009) and rejection (AHR, 1.88, 95% CI 1.16–3.05, p = 0.01) were again the only factors significantly associated with hospitalized poisonings.
Mortality after hospitalizations for poisoning, which was constant over time, is shown in Figure 4. Hospitalizations for poisonings were independently associated with increased mortality in Cox Regression (AHR, 1.54, 95% CI 1.22–1.92, p = 0.002).
Discussion
The present study demonstrated that almost all hospitalizations for poisonings after renal transplantation were due to the use of prescription medications, even though ICD9 codes 960–989.x also include poisonings from environmental toxins, vaccines, and anesthetic agents. Because many immunosuppressive medications are toxic, this was not a surprising finding. However, reports from the medical literature have been infrequent, as indicated previously. In fact, a Medline search for the terms "medication error transplant" yielded only 14 papers, [12, 13] and none was returned for the search "medication error renal transplant." Therefore, review of the medical literature might give a misleading indication of the frequency of this complication. Although reported hospitalizations for poisonings as a primary diagnosis were not common, hospitalizations for poisonings were independently associated with increased mortality in renal transplant recipients, and also represented potentially avoidable morbidity with a mean length of stay of over 4 days.
Anti-neoplastic/immunosuppressive agents were the most common cause of poisonings in this population, as would be expected. The current study almost certainly underestimates the incidence of serious immunosuppressive drug toxicity in this population. In addition, renal transplant recipients have many potential indications for the use of analgesics/antipyretics, post-operative pain among them [14]. The frequent occurrence of poisonings due to analgesics after renal transplantation may therefore seem understandable. However, although the risk of poisonings was highest in the first few months after transplantation, the increase in relative risk in this time was not visually remarkable (Figure 1), and the risk of poisoning continued in a constant fashion after transplant. There was certainly no early peak followed by a dramatic stabilization in rates of poisonings, as has been demonstrated for hospitalizations for cytomegalovirus disease, [15] contrary to what might have been expected. This suggests the possibility that either the dosing or use of these medications may not be adjusted optimally after renal transplantation.
Benzodiazepines/tranquilizers were the third most common cause of poisonings in this study. It is noteworthy that calcineurin inhibitor toxicity may mimic anxiety or nervousness, [16] which are common indications for the use of benzodiazepines. In addition, many antidepressive agents may raise levels of calcineurin inhibitors [17]. Results of the present study raise the possibility that indications for treatment with benzodiazepines may actually represent toxicities of calcineurin antagonists, which might be best managed by adjusting the dose of the calcineurin inhibitors. Until then, we would suggest that the possibility of other drug toxicities, including drug interactions, be thoroughly excluded before prescribing benzodiazepines in this population. Often overlooked is the effect of erratic compliance with azole antifungals, anticonvulsants or other medications that inhibit or induce calcineurin inhibitors.
The high frequency of poisonings due to insulin/antidiabetic agents is also not surprising given the high frequency of diabetes among renal transplant recipients, although diabetes was not an independent risk factor for poisonings in the present analysis. This suggests that diabetic transplant recipients should be monitored with particularly close attention, perhaps in concert with regular visits with a pharmacist [18] or as part of a disease management program to reduce medical errors [19].
Considering that almost all causes of hospitalized poisonings after renal transplantation were attributed to prescription medications, the risk factors for hospitalized poisonings in the present study, namely allograft rejection and low body mass index, are all the more remarkable. The significance of a low body mass index as an independent risk factor for poisonings in this analysis as a risk factor for hospitalized poisonings, independent of age, suggests the possibility of suboptimal dose adjustment of medications. This particularly applies to calcineurin inhibitors, which are lipid soluble and have a large volume of distribution. Most calcineurin inhibitors are initiated adjusted for patient weight. However, in a small percentage of patients currently recommended doses may still result in toxicity. Area-under-the-curve monitoring gives an estimate of total drug exposure and may help minimize toxicity, particularly in patients with unusual clinical responses to standard dosing [20]. It would be useful to test this hypothesis in future studies.
The association of poisonings with allograft rejection in this category of patients may represent toxicity due to bolus intravenous or high dose oral corticosteroid therapy, or significant drug-drug interactions in this setting, which the database could not determine. However, the findings of the present analysis suggest a need for providers nationally to reevaluate the use of immunosuppressive agents in a systematic fashion after transplantation. The management of renal transplant patients is certainly not uniform; in some centers, the primary transplant surgeon may continue management of the patient up to a year or more after transplant, while in other centers a nephrologist or even primary care physician may assume primary management of the patient after the first transplant month. It is possible that more standardized guidance on dosing and the possibility of drug interactions would be beneficial. Currently, there are no clinical practice guidelines on the dosing of transplant related medications, although certain institutional guidelines do exist [21].
The present study is limited by use of primary hospitalization diagnoses for outcomes. The true frequency of poisonings and medication errors in the renal transplant population is most likely underestimated. Because of the study's retrospective nature, many other factors could not be validated. Patients with multiple potential causes for admission may not have been included among patients with hospitalized poisonings. However, the study is population based, and because accurate coding for hospitalization discharge is required, is likely to be less subject to reporting bias than case reports of poisonings in the medical literature.
The present study could not determine the reason for poisonings. In short, the possibilities of patient error vs. provider error could not be distinguished. However, consultation with pharmacists may reduce both [22]. Given the looming shortage of nephrologists, [23] nurses, and other medical providers, [24] multiple safeguards need to be established to minimize the possibility of error as much as possible in these complex and challenging patients. This is particularly true given the significant associations of both body mass index and allograft rejection with hospitalized poisonings in this analysis, which may indicate that more complex and individualized methods of medication dosing may be necessary after renal transplantation than are currently used. The present study identifies specific targets for investigation and improvement.
References
Chang SH, Lim CS, Low TS, Chong HT, Tan SY: Cyclosporine-associated encephalopathy: a case report and literature review. Transplant Proc. 2001, 33: 3700-1. 10.1016/S0041-1345(01)02510-6.
Wong EH, Chan NN, Sze KH, Or KH: Serotonin syndrome in a renal transplant patient. J R Soc Med. 2002, 95: 304-5. 10.1258/jrsm.95.6.304.
Chari RS, Hemming AW, Cattral M: Successful kidney pancreas transplantation from donor with methanol intoxication. Transplantation. 1998, 66: 674-5. 10.1097/00007890-199809150-00025.
Ravishankar DK, Kashi SH, Lam FT: Organ transplantation from donor who died of cyanide poisoning: a case report. Clin Transplant. 1998, 12: 142-3.
Abbott KC, Duran M, Hypolite I, et al: Hospitalizations for bacterial endocarditis after renal transplantation in the United States. J Nephrol. 2001, 14: 353-60.
Tveit DP, Hypolite I, Bucci J, et al: Risk factors for hospitalizations resulting from pulmonary embolism after renal transplantation in the United States. J Nephrol. 2001, 14: 361-8.
Abbott KC, Hypolite IO, Hshieh P, et al: Hospitalized congestive heart failure after renal transplantation in the United States. Ann Epidemiol. 2002, 12: 115-22. 10.1016/S1047-2797(01)00272-1.
Abbott KC, Bucci JR, Cruess D, Taylor AJ, Agodoa LY: Graft loss and acute coronary syndromes after renal transplantation in the United States. J Am Soc Nephrol. 2002, 13: 2560-9.
Hypolite IO, Bucci J, Hshieh P, Cruess D, Agodoa LY, Yuan CM, Taylor AJ, Abbott KC: Acute coronary syndromes after renal transplantation in patients with end-stage renal disease resulting from diabetes. Am J Transplant. 2002, 2: 274-81. 10.1034/j.1600-6143.2002.20313.x.
David Kleinbaum: Survival Analysis :. A Self-Learning Text (Statistics in the Health Sciences). Springer Verlag; New York, New York, May 1996
Kruger HU, Bross-Bach U, Proksch B, Schmidt H, Dopfer R, Ehninger G: A case of accidental cyclosporin overdose with pharmacokinetic analysis. Bone Marrow Transplant. 1988, 3: 167-9.
Yeh CN, Hsieh CH, Hung CM, Jeng LB, Chao TC, Chen MF: Acute overdoses of tacrolimus (FK 506). Dig Dis Sci. 1999, 44: 1650-2. 10.1023/A:1026635615532.
Dines CM: Improving renal transplant pain management. Hosp Case Manag. 1999, 7: 105-7.
Abbott K, Hypolite I, Viola R, Poropatich R, Hshieh P, Cruess D, Hawkes C, Agodoa L: Hospitalizations for cytomegalovirus disease after renal transplantation in the United States. Ann Epidemiol. 2002, 12: 402-10.1016/S1047-2797(01)00283-6.
Bechstein WO: Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int. 2000, 13: 313-26. 10.1007/s001470050708.
Vella JP, Sayegh MH: Interactions between cyclosporine and newer antidepressant medications. Am J Kidney Dis. 1998, 31: 320-3.
Matzke GR, St Peter WL, Comstock TJ, Foote EF: Nephrology pharmaceutical care preceptorship: a programmatic and clinical outcomes assessment. Ann Pharmacother. 2000, 34: 593-9. 10.1345/aph.19245.
Kliger AS, Diamond LH: Patient safety in end-stage renal disease: How do we create a safe environment?. Adv Ren Replace Ther. 2001, 8: 131-7. 10.1053/jarr.2001.23991.
Wong KM, Shek CC, Chau KF, Li CS: Abbreviated Tacrolimus Area-Under-the-Curve Monitoring for Renal Transplant Recipients. Am J Kidney Dis. 2000, 35 (4): 660-666.
8/27/2002, [http://www.wramc.amedd.army.mil/departments/Medicine/Nephrology/sop/txp/txpmedprotocol.cfm]
Chisholm MA, Mulloy LL, Jagadeesan M, DiPiro JT: Impact of clinical pharmacy services on renal transplant patients' compliance with immunosuppressive medications. Clin Transplant. 2001, 15: 330-6. 10.1034/j.1399-0012.2001.150505.x.
Estimating workforce and training requirements for nephrologists through the year 2010. Ad Hoc Committee on Nephrology Manpower Needs. J Am Soc Nephrol. 1997, 8: S9-13. i–xxii, 1–32 passim
Bond MA: Health promotion and disease prevention in kidney transplant recipients. J Transpl Coord. 1998, 8: 221-4.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2369/3/10/prepub
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Authors' contributions
Kevin C. Abbott conceived the study, conducted all analysis and wrote most of the manuscript. Rebecca Viola advised on toxic effects of drugs, and wrote substantial amounts of the discussion. Lawrence Agodoa is Project Director of the NIDDK and supervised all aspects of the manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Abbott, K.C., Viola, R.A. & Agodoa, L.Y. Hospitalized poisonings after renal transplantation in the United States. BMC Nephrol 3, 10 (2002). https://doi.org/10.1186/1471-2369-3-10
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2369-3-10