Abstract
Background
We compared aortic stiffness, aortic impedance and pressure from wave reflections in the setting of bicuspid aortic valve (BAV) to the tricuspid aortic valve (TAV) in the absence of proximal aortic dilation. We hypothesized BAV is associated with abnormal arterial stiffness.
Methods
Ten BAV subjects (47 ± 4 years, 6 male) and 13 TAV subjects (46 ± 4 years, 10 male) without significant aortic valve disease were prospectively recruited. Characteristic impedance (Zc) was derived from echocardiographic images and pulse wave Doppler of the left ventricular outflow tract. Applanation tonometry was performed to obtain pulse wave velocity (PWV) at several sites as measures of arterial stiffness and augmentation index (AIx) as a measure of wave reflection.
Results
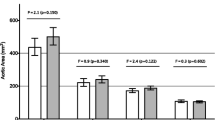
There were no significant differences between BAV and TAV subjects with regard to heart rate or blood pressure. Zc was similar between BAV and TAV subjects (p=0.25) as was carotid-femoral pulse wave velocity (cf-PWV) and carotid-radial PWV (cr-PWV) between BAV and TAV subjects (p=0.99). Carotid AIx was significantly higher in BAV patients compared with TAV patients (14.3 ± 4.18% versus -3.02 ± 3.96%, p=0.007).
Conclusions
Aortic stiffness and impedance is similar between subjects with BAV and TAV with normal aortic dimensions. The significantly higher carotid AIx in BAV, a proxy of increased pressure from wave reflections, may reflect abnormal vascular function distal to the aorta.
Similar content being viewed by others
Background
Bicuspid aortic valve (BAV) is one of the most common congenital cardiac abnormalities, occurring in 1-2% of the population [1]. A high heritability index [2] and association with variants in several genes that regulate heart development [3, 4] suggest that BAV is regulated by genetic factors. BAV is also associated with abnormalities of the thoracic aorta including dilation, aneurysm formation, coarctation, and dissection [5, 6]. More than half of young patients with normally functioning BAV have echocardiographic evidence of aortic dilatation [7], and progressive aortic dilation may develop in children [8]. The degree of aortic dilation appears out of proportion to valve dysfunction [9, 10], and aortic valve replacement does not halt the risk of subsequent aortic complications [7, 11]. Finally, aortic aneurysm can present in BAV families as an autosomal dominant trait with incomplete penetrance [12], and data suggests that genetic mutations may contribute to aortic aneurysm in the setting of a BAV [13]. Family studies, however, have not supported shared underlying genetic effects contributing to both BAV and aortic dilation [14]. Thus, evidence would support there being intrinsic abnormalities of aortic stiffness, perhaps caused by genetic factors that may invariably contribute to the risk of dilation and potential for aneurysm formation in BAV.
In this study we prospectively tested our hypothesis by measuring aortic stiffness in a cohort of BAV and TAV patients with normal proximal ascending aorta dimension. In order to further investigate vascular function in BAV, we also measured augmentation index (AIx) as a proxy of pressure from wave reflections and carotid-brachial/ carotid-radial pulse-wave velocity (cb-PWV) as indications of peripheral arterial stiffness.
Methods
Subject selection
Study subjects were prospectively recruited from the outpatient echocardiography laboratory at Tufts Medical Center. Valve morphology was assessed using two-dimensional echocardiography in standard long and short axis views. Exclusion criteria were: (i) Marfan syndrome or history of familial aortic aneurysm, (ii) internal ascending aortic diameter greater than 4.5 cm based on echocardiographic measurements, (iii) left ventricular ejection fraction <55%, (iv) moderate or severe aortic stenosis, (v) moderate or severe aortic regurgitation, (vi) aortic valve replacement, (vii) aortic coarctation, and (viii) blood pressure >160/90 mmHg. Ten consecutive BAV subjects (6 males) and 13 TAV controls (10 males) that provided consent were enrolled in the study.
Ethical clearance and consent
This study was ethically approved by the Tufts Medical Center/Tufts University Institutional Review Board after meeting the required ethical standards in compliance with the Helsinki Declaration. Every prospective participant was explained the purpose of the research and was requested to participate freely. A consent form containing details of the study regarding main purpose, role of participants, and benefits associated with participating in the study (if any) was given to each prospective participant. Those who agreed to participate in the study signed a written consent form. Patients who did not agree to participate in the study were not denied any services or treated with partiality.
Echocardiography and tonometry measurements
Subjects were studied in the supine position using a tonometry machine (Cardiovascular Engineering, Inc., Norwood, MA) and an echocardiogram machine (Philips IE33 echocardiography machine). Arterial tonometry with ECG was use to record pressure waveforms from the brachial, radial, femoral, and carotid arteries using a custom transducer as previously described [15, 16]. Two-dimensional echocardiographic images of the left ventricular outflow tract (LVOT) obtained from the parasternal long axis view were studied to measure the LVOT diameter. Pulsed Doppler of the LVOT from an apical 5-chamber view was recorded to measure LVOT blood velocity.
Calculation of Pulse Wave Velocity (PWV)
Signal averaged pressure waveforms (NiHem Noninvasive Hemodynamics Workstation v4.50, Cardiovascular Engineering) were superimposed on one another. The body surface distances between the suprasternal notch and pulse recording sites were measured using a measuring tape and calipers. PWV was calculated as the ratio of the distance (Δd) between the carotid artery and a distal arterial site to the relative time interval (Δt) between the foot of the carotid pulse wave and the distal arterial pulse wave (PWV = Δd/ Δt, cm/sec) [15, 16]. We directly measured and recorded carotid-femoral PWV (cf-PWV), carotid-brachial PWV (cb-PWV) and carotid-radial PWV (cr-PWV).
Calculation of augmentation index
The carotid pressure waveform obtained from applanation tonometry was assessed to determine peak pressure (ΔPtotal) and the inflection point created by pressure augmentation of the peripherally reflected wave (ΔPAI). The augmentation index (AIx) was computed as the ratio of augmentation pressure to peak pressure expressed as a percentage (AIx = ΔPAI/ ΔPtotal). If the inflection point occurs before peak pressure, AIx is expressed as positive percentage; if the inflection point occurs after peak pressure, AIx is expressed as a negative percentage [15]. Time to inflection is presented as a measure of wave travel timing.
Calculation of Characteristic Impedance: LVOT absolute flow (ΔV) was calculated as the product of cross sectional area, calculated from the LVOT diameter, and the LVOT blood flow velocity. The pressure differential (ΔP) created during the same time interval was estimated from ECG gated signal averaged pressure waveform acquired from applanation tonometry of the carotid artery. Characteristic impedance (Zc) was then calculated as the ratio of pressure to flow (Zc=ΔP/ΔV) [15, 16].
Calculation of arterial elastance and peak wall shear
Peak wall shear rate was calculated as 4 × (peak aortic velocity/ aortic root diameter). Cardiac dimensions were assessed using standard 2-dimensional echocardiographic techniques (Simpson’s method). Effective arterial elastance (Ea) was estimated as end systolic pressure / stroke volume and used as a measure of arterial pulsatile load related to vascular input impedance [17]. End systolic pressure was obtained from the carotid pressure waveform. Stroke volume was calculated as end-diastolic volume – end systolic volume from 2D echo.
Statistical analyses
A priori significance was set at p < 0.05. Normality of distribution was confirmed using Kolmogorov-Smirnov and Shapiro-Wilk tests. Group comparisons were made using analysis of variance for continuous variables. Chi-square tests were used to compare categorical variables. All data analysis was carried out using Statistical Package for the Social Sciences (SPSS, v 16.0, SPSS, Inc., Chicago, IL).
Results
Patient characteristics are listed in Table 1 and show that the two groups were well-matched. Both ascending aorta and aortic root size indexed to body size were similar in both groups. There was a significant difference in aortic peak velocity and wall shear rate in BAV subjects compared to TAV controls (Table 1). A total of 10 BAV patients and 13 TAV controls underwent measures of cb-PWV, cr-PWV and augmentation index. Three patients (1 BAV and 2 TAV) were excluded from the cf-PWV analysis due to poor femoral arterial waveforms. There was no statistical difference in Zc, cf-PWV, cb-PWV or cr-PWV between BAV subjects and TAV controls respectively (Table 2). Similarly, there were no group differences in effective arterial elastance (Table 2). By comparison, BAV participants were found to have significantly larger augmentation indexes compared to TAV controls (Figure 1). This difference in AIx persisted after adjusting for sex, height, and heart rate with analysis of covariance (adjusted means: 14.0% vs −2.8%, p < 0.05). Moreover, adjusting for ascending aortic diameter using a separate model also had no effect on group differences in AIx (adjusted means: 12.5% vs −1.7%, p < 0.05). AIx was significantly correlated with peak aortic velocity (r = 0.45, p = 0.02) and peak wall shear rate (r = 0.49, p < 0.05). Adjusting for either peak aortic velocity (p = 0.11) or peak wall shear rate (p = 0.12) abolished group differences in AIx.
Discussion
This study demonstrates that in the setting of a normal ascending aorta size, aortic PWV and arterial elastance appear similar between subjects with BAV and those without. By comparison, BAV participants were found to have a significantly elevated AIx compared to controls. The increased pressure from wave reflections was independent of sex, height and heart rate [18–20]. This finding is clinically significant as increased AIx is associated with increased risk of future CV events [21].
We noted elevated AIx in BAV in the absence of elevated aortic PWV and this is novel. Previous studies noting increased AIx in BAV were carried out in patients with aortic dilation [22, 23]. This is important as patients with BAV and dilated aortas have increased aortic PWV compared to BAV patients with preserved aortic size [24]. Dilation of the vessel transposes load bearing from elastin fibers to stiffer collagen fibers, increasing the incremental elastic modulus of the vessel [25, 26]. According to the Moens-Korteweg equation, this increase in elastic modulus will increase PWV. Whether increased AIx was secondary to increased aortic stiffness (from altered wave speed) or an independent phenotypic expression of this unique pathology could not be disentangled. Our findings offer important insight into the arteriopathy associated with BAV and suggest increased pressure from wave reflections as a primary systemic vascular aberration in BAV in the absence of aortic dilation, subsequent stiffening and altered reflection timing. These findings suggest a role for downstream microvascular dysfunction distal to the aorta as a potential arbitrator of increased magnitude of pressure from wave reflections in BAV.
A novel observation in the present study was the association between AIx and peak wall shear offering insight into observations of higher AIx in the setting of BAV. BAV patients with normal aortic geometry had higher wall shear than TAV due to higher aortic flow velocity and this is consistent with previous reports noted in BAV patients with dilated aortas and/or aneurysms [27, 28]. Altered valve hemodynamics have been implicated in the cause of aortic root dilation [29, 30], aneurysm [31] and valve calcification [32, 33] in BAV. Such altered central hemodynamics [34] may also damage the peripheral microvasculature [35], altering reflection sites and contributing to increased reflected pressure wave magnitude. It is interesting to note that valve replacement and normalization of aberrant central flow profiles in BAV does not mitigate aortic dilation progression [11]. Abnormalities in AIx reported herein, and elsewhere [22, 23], may reflect a previously unsuspected role for increased pressure from wave reflections in the development of large vessel arteriopathy in patients with BAV. In other patient populations such as Marfan syndrome, increased AIx has been shown to predict progression of aortic disease [36, 37]. It has been suggested that pressure pulsatility and cyclic stress from wave reflections alter load-bearing capacity of the aortic wall and contribute to fatigue-fracture (i.e. mechanical failure of biomaterials), increasing risk for aortic dilation and aneurysm [38]. The etiology of AIx as it relates to aortopathy in BAV requires further investigation.
The results of our prospective analysis finding no differences in PWV and arterial elastance between BAV and TAV patients is supported by studies showing no difference in serum matrix metalloprotein-2 levels in BAV without aneurysms compared to normal controls [24]. In addition, gene expression profiles of aorta tissue taken from BAV and TAV patients without aneurysm are very similar [39]. Our results differ from studies that demonstrated abnormal aortic root distensibility and stiffness index in BAV patients without aneurysms [40–42]. Discrepancy is likely related to method of measurement as these previous studies relied on imaging modalities such as echocardiography. Our findings are in agreement with others [22, 24] demonstrating normal aortic stiffness as measured using PWV in BAV patients without aneurysms. PWV is considered to be a robust measure of aortic stiffness and is currently viewed as the “gold standard” for measuring aortic stiffness [18–20].
We acknowledge several limitations in our study. First, since our primary hypothesis was that abnormal aortic stiffness would be found in the setting of BAV we consider our observation of increased AIx to be hypothesis generating. Second, the small size of our study may limit the generalizability of our results to the broader population of patients with BAV. We noted a partial η2 of 0.47 with an observed power of 0.79 signifying moderate effect size with adequate power for detecting group differences in AIx. Thus although possibility of a type II error exists, we believe it to be low.
Conclusions
Normal aortic stiffness with normal aortic dimension suggests that the ascending aorta is not invariably abnormal in the setting of BAV, despite the predisposition for aorta dilation and aneurysm formation. Our data should motivate further investigation into the impact of the peripheral vasculature on the central vascular phenotype in BAV patients as long term abnormalities may contribute to subsequent ascending aortic dilation and aneurysm formation in patients with BAV.
Our study demonstrates that arterial stiffness and elastance are similar between subjects with BAV and TAV with normal aortic dimensions. At the same time we demonstrate a higher carotid AIx in BAV patients consistent with abnormal vascular properties distal to the aorta. Future studies are required to determine whether abnormal distal arterial properties in BAV subjects contribute to proximal aortic dilation and aneurysm formation.
References
Braverman AC, Guven H, Beardslee MA, Makan M, Kates AM, Moon MR: The bicuspid aortic valve. Curr Probl Cardiol. 2005, 30 (9): 470-522. 10.1016/j.cpcardiol.2005.06.002.
Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW: Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004, 44 (1): 138-143. 10.1016/j.jacc.2004.03.050.
Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D: Mutations in NOTCH1 cause aortic valve disease. Nature. 2005, 437 (7056): 270-274. 10.1038/nature03940.
Wooten EC, Iyer LK, Montefusco MC, Hedgepeth AK, Payne DD, Kapur NK, Housman DE, Mendelsohn ME, Huggins GS: Application of gene network analysis techniques identifies AXIN1/PDIA2 and endoglin haplotypes associated with bicuspid aortic valve. PLoS One. 2010, 5 (1): e8830-10.1371/journal.pone.0008830.
Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J: Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002, 106 (8): 900-904. 10.1161/01.CIR.0000027905.26586.E8.
Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, Eidem B, Edwards WD, Sundt TM, Enriquez-Sarano M: Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011, 306 (10): 1104-1112. 10.1001/jama.2011.1286.
Russo CF, Mazzetti S, Garatti A, Ribera E, Milazzo A, Bruschi G, Lanfranconi M, Colombo T, Vitali E: Aortic complications after bicuspid aortic valve replacement: long-term results. Ann Thorac Surg. 2002, 74 (5): S1773-1776. 10.1016/S0003-4975(02)04261-3. discussion S1792-1779
Holmes KW, Lehmann CU, Dalal D, Nasir K, Dietz HC, Ravekes WJ, Thompson WR, Spevak PJ: Progressive dilation of the ascending aorta in children with isolated bicuspid aortic valve. Am J Cardiol. 2007, 99 (7): 978-983. 10.1016/j.amjcard.2006.10.065.
Keane MG, Wiegers SE, Plappert T, Pochettino A, Bavaria JE, Sutton MG: Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation. 2000, 102 (19 Suppl 3): III35-39.
Nistri S, Sorbo MD, Basso C, Thiene G: Bicuspid aortic valve: abnormal aortic elastic properties. J Heart Valve Dis. 2002, 11 (3): 369-373. discussion 373–364
Yasuda H, Nakatani S, Stugaard M, Tsujita-Kuroda Y, Bando K, Kobayashi J, Yamagishi M, Kitakaze M, Kitamura S, Miyatake K: Failure to prevent progressive dilation of ascending aorta by aortic valve replacement in patients with bicuspid aortic valve: comparison with tricuspid aortic valve. Circulation. 2003, 108 (Suppl 1): II291-294.
Loscalzo ML, Goh DL, Loeys B, Kent KC, Spevak PJ, Dietz HC: Familial thoracic aortic dilation and bicommissural aortic valve: a prospective analysis of natural history and inheritance. Am J Med Genet A. 2007, 143A (17): 1960-1967. 10.1002/ajmg.a.31872.
McKellar SH, Tester DJ, Yagubyan M, Majumdar R, Ackerman MJ, Sundt TM: Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2007, 134 (2): 290-296. 10.1016/j.jtcvs.2007.02.041.
Martin LJ, Hinton RB, Zhang X, Cripe LH, Benson DW: Aorta Measurements are Heritable and Influenced by Bicuspid Aortic Valve. Front Genet. 2012, 2: 61-
Mitchell GF, Izzo JL, Lacourciere Y, Ouellet JP, Neutel J, Qian C, Kerwin LJ, Block AJ, Pfeffer MA: Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation. 2002, 105 (25): 2955-2961. 10.1161/01.CIR.0000020500.77568.3C.
Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS: Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010, 122 (14): 1379-1386. 10.1161/CIRCULATIONAHA.109.914507.
Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA: Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992, 86 (2): 513-521. 10.1161/01.CIR.86.2.513.
Chemla D, Plamann K, Nitenberg A: Towards new indices of arterial stiffness using systolic pulse contour analysis: a theoretical point of view. J Cardiovasc Pharmacol. 2008, 51 (2): 111-117. 10.1097/FJC.0b013e318163a977.
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H: Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006, 27 (21): 2588-2605. 10.1093/eurheartj/ehl254.
Nichols WW: Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005, 18 (1 Pt 2): 3S-10S.
Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C: Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010, 31 (15): 1865-1871. 10.1093/eurheartj/ehq024.
Shim CY, Cho IJ, Yang WI, Kang MK, Park S, Ha JW, Jang Y, Chung N: Central aortic stiffness and its association with ascending aorta dilation in subjects with a bicuspid aortic valve. J Am Soc Echocardiogr. 2011, 24 (8): 847-852. 10.1016/j.echo.2011.04.017.
Aydin A, Mortensen K, Rybczynski M, Sheikhzadeh S, Willmann S, Bernhardt AM, Hillebrand M, Stritzke J, Baulmann J, Schunkert H: Central pulse pressure and augmentation index in asymptomatic bicuspid aortic valve disease. Int J Cardiol. 2011, 147 (3): 466-468. 10.1016/j.ijcard.2011.01.018.
Tzemos N, Lyseggen E, Silversides C, Jamorski M, Tong JH, Harvey P, Floras J, Siu S: Endothelial function, carotid-femoral stiffness, and plasma matrix metalloproteinase-2 in men with bicuspid aortic valve and dilated aorta. J Am Coll Cardiol. 2010, 55 (7): 660-668. 10.1016/j.jacc.2009.08.080.
Bank AJ, Kaiser DR: Smooth muscle relaxation: effects on arterial compliance, distensibility, elastic modulus, and pulse wave velocity. Hypertension. 1998, 32 (2): 356-359. 10.1161/01.HYP.32.2.356.
Bank AJ, Wang H, Holte JE, Mullen K, Shammas R, Kubo SH: Contribution of collagen, elastin, and smooth muscle to in vivo human brachial artery wall stress and elastic modulus. Circulation. 1996, 94 (12): 3263-3270. 10.1161/01.CIR.94.12.3263.
Barker AJ, Markl M, Burk J, Lorenz R, Bock J, Bauer S, Schulz-Menger J, von Knobelsdorff-Brenkenhoff F: Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging. 2012, 5 (4): 457-466. 10.1161/CIRCIMAGING.112.973370.
Hope MD, Hope TA, Crook SE, Ordovas KG, Urbania TH, Alley MT, Higgins CB: 4D flow CMR in assessment of valve-related ascending aortic disease. JACC Cardiovasc Imaging. 2011, 4 (7): 781-787. 10.1016/j.jcmg.2011.05.004.
Meierhofer C, Schneider EP, Lyko C, Hutter A, Martinoff S, Markl M, Hager A, Hess J, Stern H, Fratz S: Wall shear stress and flow patterns in the ascending aorta in patients with bicuspid aortic valves differ significantly from tricuspid aortic valves: a prospective study. Eur Heart J Cardiovasc Imaging. 2012, Dec 9. [Epub ahead of print]
Kim YG, Sun BJ, Park GM, Han S, Kim DH, Song JM, Kang DH, Song JK: Aortopathy and bicuspid aortic valve: haemodynamic burden is main contributor to aortic dilatation. Heart. 2012, 98 (24): 1822-1827. 10.1136/heartjnl-2012-302828.
Vergara C, Viscardi F, Antiga L, Luciani GB: Influence of bicuspid valve geometry on ascending aortic fluid dynamics: a parametric study. Artif Organs. 2012, 36 (4): 368-378. 10.1111/j.1525-1594.2011.01356.x.
Sun L, Chandra S, Sucosky P: Ex vivo evidence for the contribution of hemodynamic shear stress abnormalities to the early pathogenesis of calcific bicuspid aortic valve disease. PLoS One. 2012, 7 (10): e48843-10.1371/journal.pone.0048843.
Chandra S, Rajamannan NM, Sucosky P: Computational assessment of bicuspid aortic valve wall-shear stress: implications for calcific aortic valve disease. Biomech Model Mechanobiol. 2012, 11 (7): 1085-1096. 10.1007/s10237-012-0375-x.
Nakata M, Tatsumi E, Tsukiya T, Taenaka Y, Nishimura T, Nishinaka T, Takano H, Masuzawa T, Ohba K: Augmentative effect of pulsatility on the wall shear stress in tube flow. Artif Organs. 1999, 23 (8): 727-731. 10.1046/j.1525-1594.1999.06411.x.
Silacci P, Desgeorges A, Mazzolai L, Chambaz C, Hayoz D: Flow pulsatility is a critical determinant of oxidative stress in endothelial cells. Hypertension. 2001, 38 (5): 1162-1166. 10.1161/hy1101.095993.
Mortensen K, Aydin MA, Rybczynski M, Baulmann J, Schahidi NA, Kean G, Kuhne K, Bernhardt AM, Franzen O, Mir T: Augmentation index relates to progression of aortic disease in adults with Marfan syndrome. Am J Hypertens. 2009, 22 (9): 971-979. 10.1038/ajh.2009.115.
Mortensen K, Baulmann J, Rybczynski M, Sheikhzadeh S, Aydin MA, Treede H, Dombrowski E, Kuhne K, Peitsmeier P, Habermann CR: Augmentation index and the evolution of aortic disease in marfan-like syndromes. Am J Hypertens. 2010, 23 (7): 716-724. 10.1038/ajh.2010.78.
Payne RA: Augmenting the assessment of Marfan syndrome?. Am J Hypertens. 2009, 22 (9): 951-10.1038/ajh.2009.129.
Folkersen L, Wagsater D, Paloschi V, Jackson V, Petrini J, Kurtovic S, Maleki S, Eriksson MJ, Caidahl K, Hamsten A: Unraveling the divergent gene expression profiles in bicuspid and tricuspid aortic valve patients with thoracic aortic dilatation - the ASAP study. Mol Med. 2011, 17 (11-12): 1365-1373.
Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K: Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol. 2009, 53 (24): 2288-2295. 10.1016/j.jacc.2009.03.027.
Nistri S, Grande-Allen J, Noale M, Basso C, Siviero P, Maggi S, Crepaldi G, Thiene G: Aortic elasticity and size in bicuspid aortic valve syndrome. Eur Heart J. 2008, 29 (4): 472-479. 10.1093/eurheartj/ehm528.
Bilen E, Akcay M, Bayram NA, Kocak U, Kurt M, Tanboga IH, Bozkurt E: Aortic elastic properties and left ventricular diastolic function in patients with isolated bicuspid aortic valve. J Heart Valve Dis. 2012, 21 (2): 189-194.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2261/13/19/prepub
Acknowledgements
The authors wish to thank the members of the echocardiography laboratory for their assistance in performing these studies.
Funding
This project was supported by the American Heart Association and the National Institutes of Health grant HL069770 and HL114794.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PW recruited and consented subjects for study participation, performed tonometry experiments, assisted in data analysis and manuscript preparation. AAQ directed study implementation, assisted in data analysis and manuscript preparation, ELB directed study implementation, assisted in data analysis and manuscript preparation, AKH directed study implementation, assisted in data analysis and manuscript preparation, EP recruited and consented subjects for study participation, performed tonometry experiments, and assisted in data analysis, JTK oversaw study implementation and assisted in manuscript preparation, KSH directed study implementation, assisted in data analysis and manuscript preparation, and GSH oversaw all aspects of the study including final manuscript preparation. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Warner, P.J., Al-Quthami, A., Brooks, E.L. et al. Augmentation index and aortic stiffness in bicuspid aortic valve patients with non-dilated proximal aortas. BMC Cardiovasc Disord 13, 19 (2013). https://doi.org/10.1186/1471-2261-13-19
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2261-13-19