Abstract
Renal cell carcinoma (RCC) accounts for approximately 2.6% of all cancers in the United States. While early stage disease is curable by surgery, the median survival of metastatic disease is only 13 months. In the last decade, there has been considerable progress in understanding the genetics of RCC. The VHL tumor suppressor gene is inactivated in the majority of RCC cases. The VHL protein (pVHL) acts as an E3 ligase that targets HIF-1, the hypoxia inducible transcription factor, for degradation by the ubiquitin proteasome system (UPS). In RCC cases with mutant pVHL, HIF-1 is stabilized and aberrantly expressed in normoxia, leading to the activation of pro-survival genes such as vascular endothelial growth factor (VEGF). This review will focus on the defect in the UPS that underlies RCC and describe the development of novel therapies that target the UPS.
Publication history: Republished from Current BioData's Targeted Proteins database (TPdb; http://www.targetedproteinsdb.com).
Similar content being viewed by others
Role of the ubiquitin proteasome pathway in renal cancer
Each year in the United States, there are approximately 36,000 new cases of renal cell carcinoma (RCC) and 13,000 related deaths (statistics available at http://www.kidneycancer.org) [1]. Though there are different pathologic subtypes, the majority (~75%) of RCC cases are referred to as “conventional” or “clear cell” type (CCRCC) [1]. Greater than 95% of clear cell kidney cancers occur sporadically within the population, while the remainder occur as part of relatively rare, inherited genetic syndromes including von Hippel-Lindau disease and familial clear cell renal cancer [1, 2]. The primary genetic defect of clear cell kidney cancer (in both sporadic and hereditary forms) involves inactivation of the VHL gene pathway. Individuals with VHL disease harbor a germline mutation in one allele of the VHL gene and somatic inactivation of the remaining wild-type allele results in tumor development [3]. In sporadic CCRCC, somatic inactivation of the VHL gene also occurs in greater than 60% of cases via mutation, deletion or methylation-associated silencing [3–9]. VHL thus represents a classic tumor suppressor gene that is inactivated in CCRCC according to Knudsen's “two-hit” hypothesis [10, 11]. Indeed, loss of VHL occurs at a very early stage in kidney cancer progression, suggesting that VHL represents the “gatekeeper” gene in this malignancy [12].
For decades preceding the modern era of genetics, surgeons and pathologists had described the richly vascular nature of RCC. When the VHL gene was originally identified in 1993, however, its function was not easily deduced from its structure because the amino acid sequence of the protein (pVHL) did not share any significant homology to other known proteins at the time [13]. It was subsequently discovered, however, that pVHL negatively regulates hypoxia-inducible genes such as vascular endothelial growth factor (VEGF) and erythropoietin (EPO) in renal cancer cell lines in vitro[14, 15]. Normally, hypoxia-inducible genes such as VEGF are expressed at low or undetectable levels under normoxic conditions but are markedly induced under hypoxic conditions. In pVHL-deficient renal cancers, there is constitutive upregulation of hypoxic genes (including VEGF, erythopoietin and carbonic anhydrases) in normoxia [16–19]. Over the next decade, detailed biochemical, structural and functional analyses of pVHL identified its essential role as part of a multiprotein E3 ubiquitin ligase that targets specific proteins for destruction via the ubiquitin proteasome system (UPS) [3, 15, 20–23].
The UPS functions within normal cells of higher eukaryotes in two major ways: 1) as part of a degradative pathway that regulates the intracellular breakdown of proteins and 2) as part of a non-degradative pathway that regulates the location and activity of diverse cellular proteins [24–27]. The UPS is an integral part of normal cellular functions including cell cycle progression, signal transduction, response to extracellular stress and DNA repair (reviewed in 26) [26]. In addition, proteins that could be harmful to the cell, such as damaged, misfolded or misassembled proteins are also degraded [28].
The mechanism for proteolysis by the UPS is highly regulated and involves several steps that depend on ubiquitin, a 76 amino acid protein that is highly conserved among higher eukaryotes [29]. In the first step, the proteolysis pathway is initiated by a ubiquitin activating enzyme (E1) that uses ATP to form a high-energy thiolester bond with the C-terminus of ubiquitin [30]. In the second step, activated ubiquitin is transferred to a ubiquitin conjugating enzyme (E2) [31]. In the third step, ubiquitin is subsequently conjugated to target proteins in a process mediated by an E3 ubiquitin ligase [32]. The E3 ligase serves as an adaptor molecule that interacts with both the target protein and the E2, resulting in formation of an isopeptide bond between the C-terminus of ubiquitin and an ε-amino group of lysine residues in the target protein. Successive transfers of activated ubiquitin to lysine-48 of the previously conjugated ubiquitin molecule lead to the formation of polyubiquitin chains [33, 34], which serve as recognition markers for the 26S proteasome [35] (Figure 1).
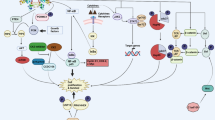
The ubiquitin proteasome system. In step 1, ubiquitin is activated by a ubiquitin activating enzyme, E1. In step 2, activated ubiquitin is transferred to a ubiquitin conjugating enzyme, E2. In step 3, ubiquitin is subsequently conjugated to target proteins in a process mediated by an E3 ubiquitin ligase. In step 4, the polyubiquitylated substrate protein is degraded by the 26S proteasome. A single E1 enzyme can transfer ubiquitin to all of the E2s in the cell, and each of the E2s associates with a restricted set of E3s that confer substrate specificity.
Conversely, while the UPS degradative pathway involves polyubiquitylation of target proteins for degradation, the non-degradative UPS pathway generally involves monoubiquitylation of proteins [36, 37]. Proteins can be modified on a single lysine with a single ubiquitin moiety or modified on multiple lysines with a single ubiquitin moiety (multi-monoubiquitylation). Examples of monoubiquitylation function include translocation of monoubiquitylated p53 to the mitochondria, endocytosis of monoubiquitylated receptor tyrosine kinases and activation of monoubiquitylated transcription factors [38–40].
The 26S proteasome is a ~2.5 MDa complex composed of a single core catalytic 20S particle capped on each end by a 19S particle [24]. The 20S particle is a barrel shaped structure comprised of four stacked rings, two outer α rings and two identical inner β rings. Each α and β ring is in turn composed of seven distinct subunits. The 20S particle has proteolytic sites facing the interior chamber of the barrel that are accessible via a narrow pore opening at either side [41]. Folded proteins, however, cannot access these pores, so native proteins have to be processed prior to degradation. Processing occurs via the 19S particle, which is comprised of at least 17 proteins ranging in molecular weight from 25 to 100 kDa. The 19S particle provides multiple functions necessary for binding, unfolding and processing of protein substrates prior to entry and degradation within the 20S particle [42, 43]. In addition, the 19S particle contains six different ATPases that harvest the energy required for proteolysis [44].
The eukaryotic 20S particle contains chymotrypsin-, trypsin-, and caspase-like proteolytic enzyme activity [45–47]. Studies utilizing specific chemical inhibitors suggest that the chymotrypsin-like activity is the most important of the three enzyme activities for proteolysis [48]. In the final step of proteolysis, polyubiquitylated target proteins bind to the 19S particle where they are deubiquitylated, unfolded, delivered into the catalytic core of the 20S particle and degraded to peptides 3–22 amino acids in size [49, 50]. The ubiquitin moieties are recycled for subsequent reactions.
Interestingly, the ubiquitin system is hierarchical: a single E1 enzyme can activate a larger number of E2s and each E2 can interact with one to several E3 proteins that ultimately tag the protein for degradation [51, 52]. Some E3 ligases are single polypeptides, while others exist as multi-protein complexes. Although the absolute number of E3 ligases in mammalian cells remains to be determined, at least several hundred E3 ligases at the end of this enzymatic cascade are thought to confer specificity to the UPS [52].
Using biochemical methods, a link between pVHL and the cellular ubiquitylation machinery was made, supported by the discovery that pVHL exists in a complex with Elongin B, Elongin C, Cul2 and Rbx1 [53–55]. This complex closely resembles SCF complexes in yeast that contain homologues including Skp1 (Elongin C), Cdc53 (Cul2), and Roc1 (Rbx1). In yeast, the SCF complex targets proteins for ubiquitin-mediated proteasomal degradation and the F-box component (Roc1) confers substrate specificity. Subsequent functional studies supported the notion that the pVHL–Elongin B–Elongin C–Cul-2–Rbx1 complex functions as an E3 ligase [20–22]. While there is little sequence homology between pVHL and an F-box protein, both have an overall pattern of hydrophobic amino acids that appears to be important for target substrate recognition [56]. These seminal observations subsequently led to the important discovery that pVHL binds to HIFα proteins and targets them for ubiquitin-mediated proteolysis [15].
In hypoxia, one of three HIFα proteins (Hif1α, Hif2α or Hif3α) associates with Hif1β to form a heterodimeric transcription factor (HIF-1) that binds to a consensus hypoxia-responsive element (HRE; 5'-RCTGTG-3') and activates a number of (greater than 60) different target genes involved in diverse biologic processes including angiogenesis, cell cycle control, proliferation and energy metabolism (reviewed in 57) [57]. While Hif1β is constitutively expressed, HIFα levels are tightly regulated in response to changes in oxygen tension. Under hypoxic conditions, pVHL does not bind to HIFα proteins, which then accumulate and bind to Hif1β to activate transcription. When cells are shifted to normoxia, however, HIFα proteins instantaneously undergo a post-translational modification resulting in hydroxylation of crucial proline residues within regions of HIFα called oxygen-dependent degradation domains (ODDs) [23, 58, 59]. Hydroxylation results in a change in conformation of the HIFα proteins, which permits binding to and degradation by pVHL. Interestingly, hydroxylation of HIFα proteins is performed by a conserved family of prolyl-4 hydroxylases that require oxygen for activity, suggesting that these enzymes contribute to oxygen sensing [60, 61].
X-ray crystallographic analysis of pVHL has revealed two major protein domains: an α domain and a β domain. The surface of the α domain (residues 155–192) is primarily responsible for the interaction between pVHL and Elongin C [56]. The surface of the β domain consists of a seven-stranded β sandwich (residues 63–154) and an α helix (residues 193–204), and is primarily responsible for binding to HIFα proteins. In sporadic RCC, about one half of VHL gene mutations map to the α domain and the other half to the β domain. The majority of these mutations (http://www.cancerindex.org/geneweb/VHL.htm) are missense mutations and many lead to aberrant upregulation of HIF-1, either by abolishing binding of pVHL to Elongin C and/or to HIFα proteins (reviewed in 3) [3, 62, 63]. In patients with inherited VHL disease, RCC tumors harbor VHL deletions or truncation mutations, also leading to aberrant upregulation of HIF-1. Taken together, these observations support a genotype-phenotype link in RCC, since the hypervascularity of these tumors can be explained by a pVHL-dependent defect in ubiquitin-mediated degradation of HIFα proteins, leading to increased HIF-1 transcriptional activity with consequent upregulation of VEGF and other factors that are thought to promote survival (reviewed in 57) [57, 64–66] (Figure 2).
Model for the E3 ligase function of pVHL in normoxia. A In normal cells, HIFα proteins are hydroxylated by prolyl-4 hydroxylases (PHDs) that require oxygen for activity. pVHL, in a complex with multiple proteins including Elongin C and Cul-2, binds to hydroxylated HIFα proteins and delivers them to the 26S proteasome for destruction. B In RCC, VHL gene mutations often disrupt pVHL–HIFα binding and/or the pVHL–Elongin C–Cul-2 complex. The consequence is that stable HIFα proteins dimerize with Hif1β and the resulting HIF-1 complex binds to a hypoxia-response element (HRE) to activate pro-survival genes, such as VEGF, EPO and Glut1.
Models for studying RCC
Most of the data described above linking pVHL function to the UPS was obtained from studies conducted in vitro. Evidence for this model evolved and converged over time from a variety of disciplines including biochemistry, structural biology and molecular genetics. Invaluable insights were gained from studies of yeast, Drosophila, Caenorhabditis elegans and human RCC cell lines [20, 54, 67–69]. Our understanding of the genotype-phenotype link in RCC is based on a thorough analysis of VHL mutations found in primary human kidney tumors [63].
Historically, there has been considerable difficulty in establishing relevant animal models for RCC. VHL-/- mice (created by the Linehan laboratory, National Cancer Institute, USA) die during early embryogenesis due to defective placental vasculogenesis [70]. VHL+/- heterozygous mice (created by the Walker laboratory, University of Texas M.D. Anderson Cancer Center, USA) are prone to vascular proliferative lesions of the liver but do not develop kidney tumors [71]. Rodents exposed to carcinogens can develop kidney tumors; however, the genetic defect involves the tumor suppressor gene Tsc-2 (tuberous sclerosis complex-2) rather than VHL and the animals display chromophilic rather than clear cell histology [71]. Interestingly, though, Tsc-2 mutant tumors, like VHL mutant tumors, are highly vascular and express very high levels of HIF-1 [72].
Several studies have generated mice with human RCC cell line-derived xenograft tumors, injected either subcutaneously or orthotopically into the kidney [69, 73–75]. While animal models have a limited capacity to recapitulate the tumor biology of humans, they have permitted controlled experiments both for the analysis of renal tumorigenesis and the evaluation of novel therapeutics for RCC. For example, when pVHL function is restored to renal cancer cell lines lacking pVHL, these cells lose the capacity to form tumors in mice in vivo[69]. However, when these same pVHL-restored RCC cells are engineered to express a stable Hif2α variant lacking its prolyl hydroxylation/pVHL binding sites, they regain their ability to form tumors in vivo[76]. These data underscore the importance of HIF-1 signaling in VHL-derived tumorigenesis.
Disease targets and ligands
The Food and Drug Administration recently approved two new agents for the treatment of advanced kidney cancer: Sutent (Sunitinib, SU011248, manufactured by Pfizer) and Nexavar (Sorafenib, Bay 43-9006, manufactured by Bayer Pharmaceuticals and Onyx). Both agents are small molecule tyrosine kinase inhibitors that block receptor signaling by primarily targeting VEGF and platelet-derived growth factor (PDGF) [77]. It appears, however, that other tyrosine kinases are targeted, for example c-Kit, which is inhibited by Sutent [78]. The aggregate effect of these drugs is to inhibit angiogenesis, although direct effects on cell proliferation may also be important. Along with Avastin (Bevacizumab, manufactured by Genentech), the anti-VEGF antibody, these agents demonstrate the therapeutic benefit of inhibiting angiogenesis in RCC [79].
The discovery that the primary genetic event in CCRCC (loss of VHL) results in a defect in the UPS suggests that novel therapies targeting this pathway could be employed to induce apoptosis in cancer cells. Importantly, transformed cells generally display increased susceptibility to apoptosis by proteasome inhibitors when compared with non-transformed cells [80]. The basis for this is under investigation, but possible explanations include stabilization of proteins that normally either contribute to apoptosis (for example p53, p21, p27, Bax and Smac/Diablo) or that antagonize pro-survival pathways (for example Iκβ, proteasomal stabilization of which inhibits nuclear translocation of NFκβ, a proto-oncogenic transcription factor) [81].
The discovery of drugs that inhibit the UPS is proceeding rapidly. To consider their potential use in RCC, it is helpful to discuss them based on the component of the UPS they target.
Inhibitors of the 26S proteasome
Bortezomib (Velcade, manufactured by Millennium Pharmaceuticals) is a dipetidyl boronic acid that reversibly inhibits the chymotryspin-like activity found within the 20S particle of the proteasome [82]. Bortezomib has already been established as an effective agent in the treatment of multiple myeloma [83]. The drug causes apoptosis of kidney cancer cell lines in vitro, but was less promising in two Phase II clinical trials [84–86]. A study from Memorial Sloan-Kettering reported four partial responses from a total group of 37 RCC patients treated (25 clear cell, six papillary, one collecting duct and one medullary), with clear cell histology present in three of the four responders [85]. A study from the University of Chicago reported one partial response in a patient with clear cell histology from a total group of 21 RCC patients treated (histologic subtypes were not fully reported) [86]. Toxicities attributed to bortezomib (including fatigue, sensory neuropathy, nausea, anemia and transaminitis) probably reflect the consequences of nonspecific inhibition of the UPS. While neither study supported the use of bortezomib as a single therapeutic agent in patients with metastatic kidney cancer, the possibility of combining it with other agents in the future could still be theoretically desirable, since proteasome inhibition can enhance chemotherapy and overcome drug resistance in preclinical models [80, 87].
Inhibitors of E3 ubiquitin ligases
As described above, E3 ubiquitin ligases are very specific in their interaction with protein substrates. Therefore, they are attractive targets for drug discovery since their inhibition would be expected to have fewer “off-target” effects and less toxicity than inhibitors of the 26S proteasome. The interaction between the p53 tumor suppressor gene and its E3 ligase, MDM2, provides an illustration of this concept. The p53 tumor suppressor pathway is inactivated in the majority of human tumors. Approximately 50% of all human tumors contain mutations in the p53 gene and the remainder (with wild-type p53) display perturbations in p53 signaling due to increased proteasomal degradation of p53 [88]. Increased proteasomal degradation of p53 occurs because of increased expression of MDM2, or alternatively, loss of Arf, an inhibitor of the p53–MDM2 interaction.
The Nutlins (cis-imidazoline derivatives, available from Sigma) are small molecule inhibitors of MDM2 that were discovered in a chemical library screen [89]. Structurally, the Nutlins occupy the p53 binding site of MDM2, thereby displacing p53 and preventing its destruction [90]. Increased levels of p53 then restore tumor suppressor function. The potential for Nutlins (which are orally bioavailable) as anti-cancer agents is now being explored in pre-clinical studies. Importantly, in tumor xenografts derived from a human osteosarcoma cell line, Nutlins suppressed tumor growth with minimal toxicity to normal tissues [91].
Development of small molecules that affect the pVHL–HIFα interaction is also a theoretic possibility. However, a conceptual dilemma immediately presents itself. In the case of p53 and MDM2, the UPS defect is inappropriately increased degradation of p53, with both proteins in their wild-type conformation. In the case of pVHL and HIFα, the UPS defect is inappropriately decreased degradation of HIFα due to mutant pVHL. In the case of p53–MDM2, the goal of a small molecule is to inhibit binding of p53 to MDM2. By contrast, in the case of pVHL–HIFα, the goal would be to promote binding of a mutant form of pVHL to HIFα. Drugs that promote, rather than inhibit, protein–protein interactions are extremely difficult to design and screen for. In addition, given the large number of VHL mutations that would presumably result in different conformations of pVHL, the development of small molecule agonists to promote a mutant pVHL–HIFα association would in reality be unfeasible.
Activators of the 26S proteasome
Since loss of pVHL E3 ligase function leads to impaired ubiquitylation and protein degradation in RCC, the development of drugs that activate proteasome function would theoretically be of interest [92]. However, a potential limitation of this strategy would be the requirement that such drugs enhance degradation of non-ubiquitylated proteins. While such non-specific “panactivators” of proteasome function have not been described, drugs are being developed that can promote ubiquitin-mediated proteasomal degradation of HIFα subunits in pVHL-deficient RCC.
Inhibitors of Hsp90
Hsp90 is described as a “super-chaperone machine” because it normally associates with certain proteins to promote their proper folding so that they can respond to a stimulus (e.g. cytosolic kinase) or bind to a ligand (e.g. a steroid hormone) [93]. Hsp90 also functions to stabilize certain proteins from proteasomal degradation. In some cases, Hsp90 stabilizes proteins that function as oncogenes in tumorigenesis, examples of which include the p210Bcr-Abl protein in chronic myelogenous leukemia, HER-2 in breast and prostate cancer, and Hif1α [94–98]. Importantly, small molecule inhibitors of Hsp90 promote degradation of HIFα proteins in a pVHL-independent manner [99, 100]. Interestingly, this process involves ubiquitylation of HIFα subunits via a mechanism that does not involve proline hydroxylation [99]. Several Phase I and/or Phase II studies are being conducted in the United States testing the Hsp90 inhibitor 17-DMAG (17-N-Allylamino-17-Demethoxygeldanamycin, available from A.G. Scientific) in patients with RCC (see http://www.cancer.gov/clinicaltrials).
Inhibitors of mTOR
The mTOR (mammalian target of rapamycin) signaling pathway has been shown to enhance HIF-1 activity in response to growth factors [101, 102]. Inhibitors of the mTOR pathway, such as rapamycin, reduce HIF-1 levels and HIF-1 transcriptional activity. The mechanism of the suppressive effect of rapamycin on HIF-1 activity is due to increased degradation of HIFα proteins by the UPS [103]. Early clinical trials have shown that the novel mTOR inhibitor CCI779 (temsirolimus, Torisel, manufactured by Wyeth Pharmaceuticals), has promising activity in patients with advanced RCC [104, 105].
Future directions in the treatment of RCC
Continued efforts to identify drugs that activate the proteasome pathway rather than inhibit it would address the fact that the primary UPS defect in RCC is its downregulation rather than upregulation. Upregulation of the UPS system has been reported in other cancer types and has been offered as one reason why these cancers are more susceptible than normal cells to apoptosis induced by proteasome inhibitors [80, 87]. While drugs that specifically target HIF-1 degradation exist, there have been no reports to date of compounds that generally increase protein turnover by the UPS. Since the pVHL E3 ligase complex targets proteins other than HIFα, for example atypical protein C, such compounds would be an interesting concept [106].
A major obstacle in treating advanced RCC tumors (i.e. those not cured by surgery) has been their resistance to most standard chemo- and radio- therapies. The reasons for this are unclear, though possibilities include the complex genetics of the tumor, defective angiogenesis and tumor hypoxia. Even Sutent and Nexavar, which have upstaged low-dose immunotherapy in the treatment of this disease, are predominantly tumoristatic rather than tumoricidal. Thus, there is considerable room for improvement in treating RCC. One prediction is that combinations of two or more drugs will likely be necessary to approach any possibility of a cure. As an example, it makes biologic sense that the combination of a drug that blocks VEGF receptor signaling, an Hsp90 inhibitor and a DNA damaging agent could synergistically promote apoptosis in RCC. However, the exact combination of drugs and the ability to administer them safely to human patients remains a daunting challenge.
Abbreviations
- UPS:
-
= ubiquitin proteasome system
- VHL:
-
= von Hippel-Lindau
- RCC:
-
= renal cell carcinoma
- CCRCC:
-
= clear cell renal cell carcinoma
- ODD:
-
= oxygen-dependent degradation domain
References
Cohen HT, McGovern FJ: Renal-cell carcinoma. N Engl J Med. 353 (23): 2477-90. 10.1056/NEJMra043172. 2005 Dec 8
Choyke PL, Glenn GM, Walther MM, Zbar B, Linehan WM: Hereditary renal cancers. Radiology. 226 (1): 33-46. 10.1148/radiol.2261011296. 2003 Jan
Kim WY, Kaelin WG: Role of VHL gene mutation in human cancer. J Clin Oncol. 22 (24): 4991-5004. 10.1200/JCO.2004.05.061. 2004 Dec 15
Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H: Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 7 (1): 85-90. 10.1038/ng0594-85. 1994 May
Shuin T, Kondo K, Torigoe S, Kishida T, Kubota Y, Hosaka M: Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 54 (11): 2852-5. 1994 Jun 1
Whaley JM, Naglich J, Gelbert L, Hsia YE, Lamiell JM, Green JS: Germ-line mutations in the von Hippel-Lindau tumor-suppressor gene are similar to somatic von Hippel-Lindau aberrations in sporadic renal cell carcinoma. Am J Hum Genet. 55 (6): 1092-102. 1994 Dec
Gallou C, Joly D, Mejean A, Staroz F, Martin N, Tarlet G, et al.: Mutations of the VHL gene in sporadic renal cell carcinoma: definition of a risk factor for VHL patients to develop an RCC. Hum Mutat. 1999, 13 (6): 464-75. 10.1002/(SICI)1098-1004(1999)13:6<464::AID-HUMU6>3.0.CO;2-A.
Kondo K, Yao M, Yoshida M, Kishida T, Shuin T, Miura T: Comprehensive mutational analysis of the VHL gene in sporadic renal cell carcinoma: relationship to clinicopathological parameters. Genes Chromosomes Cancer. 34 (1): 58-68. 10.1002/gcc.10054. 2002 May
Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S: Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A. 91 (21): 9700-4. 10.1073/pnas.91.21.9700. 1994 Oct 11
Knudson AG, Strong LC, Anderson DE: Heredity and cancer in man. Prog Med Genet. 1973, 9: 113-58.
Kaelin WG: Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2 (9): 673-82. 10.1038/nrc885. 2002 Sep
Kaelin WG: The von Hippel-Lindau tumor suppressor gene and kidney cancer. Clin Cancer Res. 10 (18 Pt 2): 6290S-5S. 2004 Sep 15
Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML: Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 260 (5112): 1317-20. 10.1126/science.8493574. 1993 May 28
Iliopoulos O, Levy AP, Jiang C, Kaelin WG, Goldberg MA: Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci U S A. 93 (20): 10595-9. 10.1073/pnas.93.20.10595. 1996 Oct 1
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME: The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 399 (6733): 271-5. 1999 May 20
Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Dvorak HF: Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 143 (5): 1255-62. 1993 Nov
Igarashi H, Esumi M, Ishida H, Okada K: Vascular endothelial growth factor overexpression is correlated with von Hippel-Lindau tumor suppressor gene inactivation in patients with sporadic renal cell carcinoma. Cancer. 95 (1): 47-53. 10.1002/cncr.10635. 2002 Jul 1
Da Silva JL, Lacombe C, Bruneval P, Casadevall N, Leporrier M, Camilleri JP: Tumor cells are the site of erythropoietin synthesis in human renal cancers associated with polycythemia. Blood. 75 (3): 577-82. 1990 Feb 1
Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A: Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 60 (24): 7075-83. 2000 Dec 15
Lonergan KM, Iliopoulos O, Ohh M, Kamura T, Conaway RC, Conaway JW: Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau tumor suppressor protein requires binding to complexes containing elongins B/C and Cul2. Mol Cell Biol. 18 (2): 732-41. 1998 Feb
Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W: The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 13 (14): 1822-33. 1999 Jul 15
Iwai K, Yamanaka K, Kamura T, Minato N, Conaway RC, Conaway JW: Identification of the von Hippel-lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Natl Acad Sci U S A. 96 (22): 12436-41. 10.1073/pnas.96.22.12436. 1999 Oct 26
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M: HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 292 (5516): 464-8. 2001 Apr 20
Glickman MH, Ciechanover A: The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 82 (2): 373-428. 2002 Apr
Burger AM, Seth AK: The ubiquitin-mediated protein degradation pathway in cancer: therapeutic implications. Eur J Cancer. 40 (15): 2217-29. 10.1016/j.ejca.2004.07.006. 2004 Oct
Hoeller D, Hecker CM, Dikic I: Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat Rev Cancer. 6 (10): 776-88. 10.1038/nrc1994. 2006 Oct
Reinstein E, Ciechanover A: Narrative review: protein degradation and human diseases: the ubiquitin connection. Ann Intern Med. 145 (9): 676-84. 2006 Nov 7
McDonough H, Patterson C: CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones. 2003, 8 (4): 303-8. 10.1379/1466-1268(2003)008<0303:CALBTC>2.0.CO;2.
Wilkinson KD, Urban MK, Haas AL: Ubiquitin is the ATP-dependent proteolysis factor I of rabbit reticulocytes. J Biol Chem. 255 (16): 7529-32. 1980 Aug 25
Handley PM, Mueckler M, Siegel NR, Ciechanover A, Schwartz AL: Molecular cloning, sequence, and tissue distribution of the human ubiquitin-activating enzyme E1. Proc Natl Acad Sci U S A. 88 (1): 258-62. 10.1073/pnas.88.1.258. 1991 Jan 1
Hershko A, Heller H, Elias S, Ciechanover A: Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem. 258 (13): 8206-14. 1983 Jul 10
Hershko A, Heller H, Eytan E, Reiss Y: The protein substrate binding site of the ubiquitin-protein ligase system. J Biol Chem. 261 (26): 11992-9. 1986 Sep 15
Hershko A, Ciechanover A, Heller H, Haas AL, Rose IA: Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A. 77 (4): 1783-6. 10.1073/pnas.77.4.1783. 1980 Apr
Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK: A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 243 (4898): 1576-83. 10.1126/science.2538923. 1989 Mar 24
Thrower JS, Hoffman L, Rechsteiner M, Pickart CM: Recognition of the polyubiquitin proteolytic signal. EMBO J. 19 (1): 94-102. 10.1093/emboj/19.1.94. 2000 Jan 4
Hicke L: Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2 (3): 195-201. 10.1038/35056583. 2001 Mar
Haglund K, Di Fiore PP, Dikic I: Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci. 28 (11): 598-603. 10.1016/j.tibs.2003.09.005. 2003 Nov
Marchenko ND, Wolff S, Erster S, Becker K, Moll UM: Monoubiquitylation promotes mitochondrial p53 translocation. EMBO J. 2007 Feb 1
Mosesson Y, Shtiegman K, Katz M, Zwang Y, Vereb G, Szollosi J: Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J Biol Chem. 278 (24): 21323-6. 10.1074/jbc.C300096200. 2003 Jun 13
van der HA, de Vries-Smits AM, Brenkman AB, van Triest MH, van den BN, Colland F: FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol. 8 (10): 1064-73. 10.1038/ncb1469. 2006 Oct
Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik HD: Structure of 20S proteasome from yeast at 2.4 A resolution. Nature. 386 (6624): 463-71. 10.1038/386463a0. 1997 Apr 3
DeMartino GN, Proske RJ, Moomaw CR, Strong AA, Song X, Hisamatsu H: Identification, purification, and characterization of a PA700-dependent activator of the proteasome. J Biol Chem. 271 (6): 3112-8. 1996 Feb 9
Ferrell K, Wilkinson CR, Dubiel W, Gordon C: Regulatory subunit interactions of the 26S proteasome, a complex problem. Trends Biochem Sci. 25 (2): 83-8. 10.1016/S0968-0004(99)01529-7. 2000 Feb
Neuwald AF, Aravind L, Spouge JL, Koonin EV: AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9 (1): 27-43. 1999 Jan
Rivett AJ: The multicatalytic proteinase. Multiple proteolytic activities. J Biol Chem. 264 (21): 12215-9. 1989 Jul 25
Heinemeyer W, Fischer M, Krimmer T, Stachon U, Wolf DH: The active sites of the eukaryotic 20 S proteasome and their involvement in subunit precursor processing. J Biol Chem. 272 (40): 25200-9. 10.1074/jbc.272.40.25200. 1997 Oct 3
Groll M, Heinemeyer W, Jager S, Ullrich T, Bochtler M, Wolf DH: The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study. Proc Natl Acad Sci U S A. 96 (20): 10976-83. 10.1073/pnas.96.20.10976. 1999 Sep 28
Gardner RC, Assinder SJ, Christie G, Mason GG, Markwell R, Wadsworth H: Characterization of peptidyl boronic acid inhibitors of mammalian 20 S and 26 S proteasomes and their inhibition of proteasomes in cultured cells. Biochem J. 346 (Pt 2): 447-54. 2000 Mar 1
Kisselev AF, Akopian TN, Goldberg AL: Range of sizes of peptide products generated during degradation of different proteins by archaeal proteasomes. J Biol Chem. 273 (4): 1982-9. 10.1074/jbc.273.4.1982. 1998 Jan 23
Kisselev AF, Akopian TN, Woo KM, Goldberg AL: The sizes of peptides generated from protein by mammalian 26 and 20 S proteasomes. Implications for understanding the degradative mechanism and antigen presentation. J Biol Chem. 274 (6): 3363-71. 10.1074/jbc.274.6.3363. 1999 Feb 5
Semple CA: The comparative proteomics of ubiquitination in mouse. Genome Res. 13 (6B): 1389-94. 2003 Jun
Wong BR, Parlati F, Qu K, Demo S, Pray T, Huang J: Drug discovery in the ubiquitin regulatory pathway. Drug Discov Today. 8 (16): 746-54. 10.1016/S1359-6446(03)02780-6. 2003 Aug 15
Kibel A, Iliopoulos O, DeCaprio JA, Kaelin WG: Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 269 (5229): 1444-6. 10.1126/science.7660130. 1995 Sep 8
Pause A, Lee S, Worrell RA, Chen DY, Burgess WH, Linehan WM: The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc Natl Acad Sci U S A. 94 (6): 2156-61. 10.1073/pnas.94.6.2156. 1997 Mar 18
Kamura T, Koepp DM, Conrad MN, Skowyra D, Moreland RJ, Iliopoulos O: Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 284 (5414): 657-61. 10.1126/science.284.5414.657. 1999 Apr 23
Stebbins CE, Kaelin WG, Pavletich NP: Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science. 284 (5413): 455-61. 10.1126/science.284.5413.455. 1999 Apr 16
Semenza GL: Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 3 (10): 721-32. 10.1038/nrc1187. 2003 Oct
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ: Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 292 (5516): 468-72. 2001 Apr 20
Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, Pugh CW: Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature. 417 (6892): 975-8. 10.1038/nature00767. 2002 Jun 27
Bruick RK, McKnight SL: A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 294 (5545): 1337-40. 10.1126/science.1066373. 2001 Nov 9
Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J: HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 22 (16): 4082-90. 10.1093/emboj/cdg392. 2003 Aug 15
Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE: Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2 (7): 423-7. 2000 Jul
Ong KR, Woodward ER, Killick P, Lim C, Macdonald F, Maher ER: Genotype-phenotype correlations in von Hippel-Lindau disease. Hum Mutat. 2006 Oct 5
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M: Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 394 (6692): 485-90. 10.1038/28867. 1998 Jul 30
Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ: Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 94 (15): 8104-9. 10.1073/pnas.94.15.8104. 1997 Jul 22
Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM: Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 60 (15): 4010-5. 2000 Aug 1
Aso T, Yamazaki K, Aigaki T, Kitajima S: Drosophila von Hippel-Lindau tumor suppressor complex possesses E3 ubiquitin ligase activity. Biochem Biophys Res Commun. 276 (1): 355-61. 10.1006/bbrc.2000.3451. 2000 Sep 16
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR: C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 107 (1): 43-54. 10.1016/S0092-8674(01)00507-4. 2001 Oct 5
Iliopoulos O, Kibel A, Gray S, Kaelin WG: Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1 (8): 822-6. 10.1038/nm0895-822. 1995 Aug
Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A: Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci U S A. 94 (17): 9102-7. 10.1073/pnas.94.17.9102. 1997 Aug 19
Kleymenova E, Everitt JI, Pluta L, Portis M, Gnarra JR, Walker CL: Susceptibility to vascular neoplasms but no increased susceptibility to renal carcinogenesis in Vhl knockout mice. Carcinogenesis. 25 (3): 309-15. 10.1093/carcin/bgh017. 2004 Mar
Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG: TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 4 (2): 147-58. 10.1016/S1535-6108(03)00187-9. 2003 Aug
Gnarra JR, Zhou S, Merrill MJ, Wagner JR, Krumm A, Papavassiliou E: Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc Natl Acad Sci U S A. 93 (20): 10589-94. 10.1073/pnas.93.20.10589. 1996 Oct 1
Schoenfeld A, Davidowitz EJ, Burk RD: A second major native von Hippel-Lindau gene product, initiated from an internal translation start site, functions as a tumor suppressor. Proc Natl Acad Sci U S A. 95 (15): 8817-22. 10.1073/pnas.95.15.8817. 1998 Jul 21
Naito S, von Eschenbach AC, Fidler IJ: Different growth pattern and biologic behavior of human renal cell carcinoma implanted into different organs of nude mice. J Natl Cancer Inst. 78 (2): 377-85. 1987 Feb
Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG: Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 1 (3): 237-46. 10.1016/S1535-6108(02)00043-0. 2002 Apr
Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G: In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 9 (1): 327-37. 2003 Jan
Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM: SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2 (5): 471-8. 2003 May
Rathmell WK, Wright TM, Rini BI: Molecularly targeted therapy in renal cell carcinoma. Expert Rev Anticancer Ther. 5 (6): 1031-40. 10.1586/14737140.5.6.1031. 2005 Dec
Voorhees PM, Dees EC, O'Neil B, Orlowski RZ: The proteasome as a target for cancer therapy. Clin Cancer Res. 9 (17): 6316-25. 2003 Dec 15
Traenckner EB, Wilk S, Baeuerle PA: A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. EMBO J. 13 (22): 5433-41. 1994 Nov 15
Groll M, Berkers CR, Ploegh HL, Ovaa H: Crystal structure of the boronic acid-based proteasome inhibitor bortezomib in complex with the yeast 20S proteasome. Structure. 14 (3): 451-6. 10.1016/j.str.2005.11.019. 2006 Mar
Kane RC, Farrell AT, Sridhara R, Pazdur R: United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res. 12 (10): 2955-60. 10.1158/1078-0432.CCR-06-0170. 2006 May 15
An J, Sun Y, Fisher M, Rettig MB: Maximal apoptosis of renal cell carcinoma by the proteasome inhibitor bortezomib is nuclear factor-kappaB dependent. Mol Cancer Ther. 3 (6): 727-36. 2004 Jun
Kondagunta GV, Drucker B, Schwartz L, Bacik J, Marion S, Russo P: Phase II trial of bortezomib for patients with advanced renal cell carcinoma. J Clin Oncol. 22 (18): 3720-5. 10.1200/JCO.2004.10.155. 2004 Sep 15
Davis NB, Taber DA, Ansari RH, Ryan CW, George C, Vokes EE: Phase II trial of PS-341 in patients with renal cell cancer: a University of Chicago phase II consortium study. J Clin Oncol. 22 (1): 115-9. 10.1200/JCO.2004.07.165. 2004 Jan 1
Voorhees PM, Orlowski RZ: The proteasome and proteasome inhibitors in cancer therapy. Annu Rev Pharmacol Toxicol. 2006, 46: 189-213. 10.1146/annurev.pharmtox.46.120604.141300.
Vousden KH, Prives C: P53 and prognosis: new insights and further complexity. Cell. 120 (1): 7-10. 2005 Jan 14
Nalepa G, Rolfe M, Harper JW: Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov. 5 (7): 596-613. 10.1038/nrd2056. 2006 Jul
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z: In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 303 (5659): 844-8. 10.1126/science.1092472. 2004 Feb 6
Yang Y, Ludwig RL, Jensen JP, Pierre SA, Medaglia MV, Davydov IV: Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell. 7 (6): 547-59. 10.1016/j.ccr.2005.04.029. 2005 Jun
Gorospe M, Egan JM, Zbar B, Lerman M, Geil L, Kuzmin I: Protective function of von Hippel-Lindau protein against impaired protein processing in renal carcinoma cells. Mol Cell Biol. 19 (2): 1289-300. 1999 Feb
Whitesell L, Lindquist SL: HSP90 and the chaperoning of cancer. Nat Rev Cancer. 5 (10): 761-72. 10.1038/nrc1716. 2005 Oct
An WG, Schulte TW, Neckers LM: The heat shock protein 90 antagonist geldanamycin alters chaperone association with p210bcr-abl and v-src proteins before their degradation by the proteasome. Cell Growth Differ. 11 (7): 355-60. 2000 Jul
Chavany C, Mimnaugh E, Miller P, Bitton R, Nguyen P, Trepel J: p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J Biol Chem. 271 (9): 4974-7. 1996 Mar 1
Mimnaugh EG, Chavany C, Neckers L: Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem. 271 (37): 22796-801. 1996 Sep 13
Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D: 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 8 (5): 986-93. 2002 May
Minet E, Mottet D, Michel G, Roland I, Raes M, Remacle J: Hypoxia-induced activation of HIF-1: role of HIF-1alpha-Hsp90 interaction. FEBS Lett. 460 (2): 251-6. 10.1016/S0014-5793(99)01359-9. 1999 Oct 29
Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM: Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 277 (33): 29936-44. 10.1074/jbc.M204733200. 2002 Aug 16
Isaacs JS, Xu W, Neckers L: Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 3 (3): 213-7. 10.1016/S1535-6108(03)00029-1. 2003 Mar
Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM: Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 60 (6): 1541-5. 2000 Mar 15
Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL: Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 277 (41): 38205-11. 10.1074/jbc.M203781200. 2002 Oct 11
Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F: Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 22 (20): 7004-14. 10.1128/MCB.22.20.7004-7014.2002. 2002 Oct
Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR: Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 22 (5): 909-18. 10.1200/JCO.2004.08.185. 2004 Mar 1
Thomas GV, Tran C, Mellinghoff IK, Welsbie DS, Chan E, Fueger B: Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 12 (1): 122-7. 10.1038/nm1337. 2006 Jan
Ohh M: Ubiquitin pathway in VHL cancer syndrome. Neoplasia. 8 (8): 623-9. 10.1593/neo.06442. 2006 Aug
Publication history
Republished from Current BioData's Targeted Proteins database (TPdb; http://www.targetedproteinsdb.com).
Acknowledgements
This article has been published as part of BMC Biochemistry Volume 8 Supplement 1, 2007: Ubiquitin-Proteasome System in Disease Part 1. The full contents of the supplement are available online at http://www.biomedcentral.com/1471-2091/8?issue=S1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Corn, P.G. Role of the ubiquitin proteasome system in renal cell carcinoma. BMC Biochem 8 (Suppl 1), S4 (2007). https://doi.org/10.1186/1471-2091-8-S1-S4
Published:
DOI: https://doi.org/10.1186/1471-2091-8-S1-S4