Abstract
Translesion synthesis (TLS) is an error-prone pathway required to overcome replication blockage by DNA damage. Aberrant activation of TLS has been suggested to play a role in tumorigenesis by promoting genetic mutations. However, the precise molecular mechanisms underlying TLS-mediated tumorigenesis in vivo remain unclear. Rev1 is a member of the Y family polymerases and plays a key role in the TLS pathway. Here we introduce the existing to date Rev1-mutated mouse models, including the Rev1 transgenic (Tg) mouse model generated in our laboratory. We give an overview of the current knowledge on how different disruptions in Rev1 functions impact mutagenesis and the suggested molecular mechanisms underlying these effects. We summarize the available data from ours and others’ in vivo studies on the role of Rev1 in the initiation and promotion of cancer, emphasizing how Rev1-mutated mouse models can be used as complementary tools for future research.
Similar content being viewed by others
Introduction
The genome of all living organisms is continually attacked by endogenous and exogenous genotoxic DNA damaging agents, such as metabolic processes, UV light, ionizing radiation, etc., and various DNA repair pathways have evolved to protect genome integrity. TLS is a mechanism of DNA damage tolerance involving specialized DNA polymerases with the capacity to replicate across damaged DNA template [1,2,3,4,5]. Mammalian TLS polymerases include Y-family polymerases (Pol η, Pol ι, Pol κ, and Rev1), 1 B-family polymerase (Pol ζ: Rev3L/ Rev7/ PolD2/ PolD3), 2 A-family polymerases (Pol θ and Pol ν), and three X-family polymerases (Pol μ, Pol λ, and Pol β) [6].
Rev1, in tandem with Pol ζ, stands out as a central player in error-prone TLS [3, 4, 7]. Rev1 is a deoxycytidyl transferase which incorporates deoxycytosines opposite structurally diverse damaged nucleotides, such as 6O-meG, a guanine with a large adduct at the C8 or N2 position, or abasic sites [8,9,10,11,12,13,14,15,16,17]. However, the most important function of Rev1 in error-prone TLS is regulatory rather than catalytic by recruiting the Y-family polymerases Pol η, Pol ι and Pol κ and the B-family Pol ζ, which interacts with Rev1 via its Rev7 component [18,19,20,21,22,23]. Inhibition of Rev1 results in enhanced sensitivity and reduced mutation frequencies in response to DNA-damaging agents, such as UV light, hydrogen peroxide, cisplatin, and X-rays [24,25,26,27,28].
Mutations in human REV1 have been detected in a minority of tumors [29], and single-nucleotide polymorphisms (SNPs) in the hREV1 gene have been linked to various types of human cancer [30,31,32]. As with other TLS polymerases, upregulation of hREV1 is associated with the pathogenesis of human cancer [33]. Since hREV1 plays a critical role in TLS, and TLS contributes to the pathogenesis of tumors and to drug resistance by promoting tolerance of DNA damage, targeting hREV1 might be a promising approach for improving the outcome of chemotherapy. Recently, Wojtaszek J et al. reported that a small-molecule inhibitor of mutagenic translesion DNA synthesis (JH-RE-06), which disrupts the interaction between hREV1 and/or REV7, sensitizes cancer cells to cisplatin in vitro and in vivo [34].
Currently, despite the fact that several Rev1-mutated mouse models have been established, there is very little data regarding the role of Rev1 dysregulations in carcinogenesis in vivo (Table 1). Here we introduce four complementary Rev1 mutated mouse models, including the Rev1 Tg mouse model generated in our laboratory, and we discuss their distinctive advantages for carcinogenesis research. This is the review based on the authors’ presentation at the Open Symposium of the Japanese Environmental Mutagen Society (JEMS) in 2017 [35].
Rev1 KO mice
Rev1 knockout (KO) mice were first generated with the objective of investigating the role of Rev1 in somatic hypermutation (SHM) [36]. The authors reported that Rev1 KO mice showed delayed growth, a shortened life-span and were nearly infertile [36]. It should be noted that in addition to SHM, Rev1 is also involved in class switch DNA recombination (CSR), and both SHM and CSR are critical for maturation of the antibody response [37,38,39]. SHM and CSR are initiated by activation-induced cytidine deaminase (AID), which deaminates deoxycytosine (dC) residues to yield deoxyuridine (dU): deoxyguanine (dG) mispairs. These mispairs then trigger DNA repair processes facilitated by Uracil DNA glycosylase (Ung), Mismatch repair (MMR) proteins, and Rev1 [40]. SHM analysis demonstrated that mutation frequency and distribution were similar in B-cells from Rev1 KO and wild type mice [36]. In contrast, the mutation spectra were significantly altered by the deletion of Rev1. An almost complete absence of C to G transversions was observed in Rev1 KO cells, accompanied by a moderate decrease in G to C transversions and an increase in A to T substitutions similar to Ung deficiency. Since the induction of A to T mutations is highly dependent on Pol η, it is possible that the Rev1 defect results in increased access of Pol η to sites of DNA lesions. It has been reported that G: C to C: G transversions during SHM are generated downstream of two pathways: Ung2-dependent/Msh2-independent and Ung2/Msh2-dependent pathways [41]. Rev1 is indispensable in the former pathway and it plays the main role in the latter pathway, although in this case it can be replaced by other TLS polymerases. During CSR Rev1 recruits Ung to switch (S) regions and enhances dU glycosylation [42]. Nevertheless, Rev1 deficiency only slightly reduces CSR. In contrast, double Rev1/Msh2 defects lead to ablation of CSR, similarly to double Ung/Msh2 deficiency. CSR analysis has revealed that Rev1 exerts its functions in CSR via its non-catalytic properties.
Mouse embryonic fibroblasts (MEF) derived from Rev1 KO mice have been reported to proliferate more poorly than wild-type cells [43]. Similarly, Rev1 KO hematopoietic stem cells display competitive and proliferative disadvantage [44]. Furthermore, the additional disruption of Xpc, which is essential for global-genome nucleotide excision repair (ggNER), results in progressive loss of bone marrow, and fatal aplastic anemia between 3 and 4 months of age [44]. This finding suggests that Rev1-dependent TLS protects the genomic and functional integrity of the hematopoietic system in coordination with ggNER. In summary, the currently existing data is limited to genome instability and mutagenesis, but carcinogenesis studies on Rev1 KO mice have not been published, and the impact of Rev1 deficiency on cancer development in vivo remains unknown.
Rev1AA mice
Rev1AA mice are defective specifically for Rev1 catalytic activity due to mutations in a Y-family DNA polymerase catalytic domain of Rev1 (Fig. 1) [45]. Rev1AA mice develop normally and are fertile. SHM analysis has demonstrated that B-cells from Rev1AA mice are characterized by reduced overall mutation frequency and decreased mutagenesis at both G:C and A:T base pairs. This contrasts the abovementioned increase in A to T substitutions in Rev1 KO mice, and one likely explanation is that Rev1AA might inhibit the access of Pol η to sites of DNA lesions by remaining at the abasic site. Carcinogenesis study conducted in our laboratory suggests that Rev1AA does not affect chemically-induced mutagenesis and carcinogenesis (Sasatani et al., manuscript in preparation). Thus, the catalytic domain of Rev1 appears to be dispensable for either normal development or tumorigenesis.
Rev1 BRCA1 C terminus (BRCT) mice
Rev1BRCT mice carry a deletion in the BRCT domain of Rev1, which, however, retains catalytic function (Fig. 1) [46]. Rev1BRCT exhibit a normal phenotype spontaneously, while being sensitive to exogenous DNA-damaging factors and exhibiting lower levels of ultraviolet C (UVC)-induced mutagenesis. Interestingly, despite the reduced mutagenesis, RevBRCT;Xpc KO mice have been shown to be more vulnerable to skin carcinogenesis than Xpc KO mice. The authors concluded that this paradoxical phenotype was due to the induction of inflammatory hyperplasia that facilitates tumor promotion. Thus, the Rev1BRCT mouse model is useful for interrogating the distinctive roles of Rev1 in the initiation versus the promotion step of tumor development.
Rev1 Tg mice
Rev1 Tg mice were generated in our laboratory by using the metallothionein promoter 1 (MT-1) to achieve inducible expression [47]. We found that the Rev1 transgene was expressed at low levels in the liver and kidney, but at dramatically higher levels in the thymus, spleen, and lymph nodes. In order to determine whether overexpression of Rev1 would influence spontaneous tumor initiation and progression, we monitored cohorts of Wt and Rev1 Tg mice over their lifespan (> 2 years). No significant effect on overall survival or tumor incidence was observed, suggesting that overexpression of Rev1 by itself is not sufficient to stimulate tumorigenesis. However, our study revealed that overexpression of Rev1 promotes development of chemically-induced tumors, namely azoxymethane (AOM)-induced hepatocellular carcinoma, and N-methyl-N-nitrosourea (MNU)-induced thymic lymphoma and intestinal adenomas (Sasatani et al., manuscript in preparation) [47, 48]. Furthermore, in a comparative analysis of MNU-induced carcinogenesis in Rev1 Tg (Homo) mice, which are homozygous (Tg+/Tg+) for the Rev1 transgene, versus heterozygous Rev1 Tg mice, we provided evidence that Rev1 overexpression accelerates tumorigenesis in proportion to the Rev1 expression level. Following MNU treatment, we observed enhanced mutagenesis and suppressed apoptosis in proportion to the level of Rev1 overexpression.
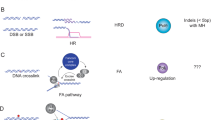
Our data implies that overexpression of Rev1 promotes mutagenic TLS to safeguard replication on damaged templates, consequently inhibiting apoptosis and accelerating tumorigenesis (Fig. 2) (Table 2). Although the role of REV1 overexpression in human carcinogenesis remains poorly understood, human cells overexpressing REV1 exhibit a comparable to the abovementioned phenotype, with enhanced mutation frequency and hindered cell death, therefore, it can be clearly stated that regulation of Rev1 levels is required for maintaining genomic stability and tumor suppression (Table 2) [47].
Model of accelerated chemically-induced tumorigenesis mediated by Rev1 overexpression. Overexpressed Rev1 suppresses apoptosis and increases the mutation frequency after the treatment of chemical reagents. The surviving fraction of mutated cells was higher under Rev1 overexpression, resulting in acceleration of carcinogenesis
Conclusions
Rev1 is a member of the TLS polymerase family and plays a key role in this mutagenic pathway, which allows the bypass of modified DNA bases and respectively, facilitates proliferation even in the presence of extensive DNA damage, such as during chemotherapy. Therefore, inhibition of the TLS pathway may be a promising strategy to tackle the problem of resistance to chemotherapy and to improve the therapeutic outcome.
Here we have introduced several mouse models with disruptions in Rev1 functions, including the Rev1 Tg mouse model generated in our laboratory. Recently we have reported that in Rev1 Tg mice the elevated Rev1 expression allows cells with mutations to survive after DNA damages, resulting in an acceleration of tumorigenesis [47, 48]. However, in vivo data like this is almost completely absent from the literature. We hope that data from studies employing Rev1-mutated mouse models will soon become available and will help us elucidate the mechanisms of Rev1-mediated tumorigenesis and chemotherapy resistance, so that we can in the future harness the therapeutic potential of TLS targeting.
Availability of data and materials
Not applicable.
Abbreviations
- Rev1:
-
Reversionless 1
- SHM:
-
Somatic hypermutation
- Tg:
-
Transgenic
- TLS:
-
Translesion synthesis
References
Friedberg EC, Wagner R, Radman M. Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science. 2002;296(5573):1627–30.
Ohmori H, Friedberg EC, Fuchs RP, Goodman MF, Hanaoka F, Hinkle D, et al. The Y-family of DNA polymerases. Mol Cell. 2001;8(1):7–8.
Lawrence CW. Cellular roles of DNA polymerase zeta and Rev1 protein. DNA Repair (Amst). 2002;1(6):425–35.
Lawrence CW. Cellular functions of DNA polymerase zeta and Rev1 protein. Adv Protein Chem. 2004;69:167–203.
Yang W, Gao Y. Translesion and repair DNA polymerases: diverse structure and mechanism. Annu Rev Biochem. 2018;87:239–61.
Yamanaka K, Chatterjee N, Hemann MT, Walker GC. Inhibition of mutagenic translesion synthesis: a possible strategy for improving chemotherapy? PLoS Genet. 2017;13(8):e1006842.
Gan GN, Wittschieben JP, Wittschieben BO, Wood RD. DNA polymerase zeta (pol zeta) in higher eukaryotes. Cell Res. 2008;18(1):174–83.
Lin W, Xin H, Zhang Y, Wu X, Yuan F, Wang Z. The human REV1 gene codes for a DNA template-dependent dCMP transferase. Nucleic Acids Res. 1999;27(22):4468–75.
Nelson JR, Lawrence CW, Hinkle DC. Deoxycytidyl transferase activity of yeast REV1 protein. Nature. 1996;382(6593):729–31.
Masuda Y, Takahashi M, Fukuda S, Sumii M, Kamiya K. Mechanisms of dCMP transferase reactions catalyzed by mouse Rev1 protein. J Biol Chem. 2002;277(4):3040–6.
Masuda Y, Kamiya K. Biochemical properties of the human REV1 protein. FEBS Lett. 2002;520(1–3):88–92.
Masuda Y, Takahashi M, Tsunekuni N, Minami T, Sumii M, Miyagawa K, et al. Deoxycytidyl transferase activity of the human REV1 protein is closely associated with the conserved polymerase domain. J Biol Chem. 2001;276(18):15051–8.
Zhang Y, Wu X, Rechkoblit O, Geacintov NE, Taylor JS, Wang Z. Response of human REV1 to different DNA damage: preferential dCMP insertion opposite the lesion. Nucleic Acids Res. 2002;30(7):1630–8.
Washington MT, Minko IG, Johnson RE, Haracska L, Harris TM, Lloyd RS, et al. Efficient and error-free replication past a minor-groove N2-guanine adduct by the sequential action of yeast Rev1 and DNA polymerase zeta. Mol Cell Biol. 2004;24(16):6900–6.
Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Protein-template-directed synthesis across an acrolein-derived DNA adduct by yeast Rev1 DNA polymerase. Structure. 2008;16(2):239–45.
Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. DNA synthesis across an abasic lesion by yeast REV1 DNA polymerase. J Mol Biol. 2011;406(1):18–28.
Piao J, Masuda Y, Kamiya K. Specific amino acid residues are involved in substrate discrimination and template binding of human REV1 protein. Biochem Biophys Res Commun. 2010;392(2):140–4.
Guo C, Fischhaber PL, Luk-Paszyc MJ, Masuda Y, Zhou J, Kamiya K, et al. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J. 2003;22(24):6621–30.
Ohashi E, Murakumo Y, Kanjo N, Akagi J, Masutani C, Hanaoka F, et al. Interaction of hREV1 with three human Y-family DNA polymerases. Genes Cells. 2004;9(6):523–31.
Tissier A, Kannouche P, Reck MP, Lehmann AR, Fuchs RP, Cordonnier A. Co-localization in replication foci and interaction of human Y-family members, DNA polymerase pol eta and REVl protein. DNA Repair (Amst). 2004;3(11):1503–14.
Ohashi E, Hanafusa T, Kamei K, Song I, Tomida J, Hashimoto H, et al. Identification of a novel REV1-interacting motif necessary for DNA polymerase kappa function. Genes Cells. 2009;14(2):101–11.
Murakumo Y, Ogura Y, Ishii H, Numata S, Ichihara M, Croce CM, et al. Interactions in the error-prone postreplication repair proteins hREV1, hREV3, and hREV7. J Biol Chem. 2001;276(38):35644–51.
Masuda Y, Ohmae M, Masuda K, Kamiya K. Structure and enzymatic properties of a stable complex of the human REV1 and REV7 proteins. J Biol Chem. 2003;278(14):12356–60.
Okada T, Sonoda E, Yoshimura M, Kawano Y, Saya H, Kohzaki M, et al. Multiple roles of vertebrate REV genes in DNA repair and recombination. Mol Cell Biol. 2005;25(14):6103–11.
Ross AL, Simpson LJ, Sale JE. Vertebrate DNA damage tolerance requires the C-terminus but not BRCT or transferase domains of REV1. Nucleic Acids Res. 2005;33(4):1280–9.
Okuda T, Lin X, Trang J, Howell SB. Suppression of hREV1 expression reduces the rate at which human ovarian carcinoma cells acquire resistance to cisplatin. Mol Pharmacol. 2005;67(6):1852–60.
Clark DR, Zacharias W, Panaitescu L, McGregor WG. Ribozyme-mediated REV1 inhibition reduces the frequency of UV-induced mutations in the human HPRT gene. Nucleic Acids Res. 2003;31(17):4981–8.
Gibbs PE, Wang XD, Li Z, McManus TP, McGregor WG, Lawrence CW, et al. The function of the human homolog of Saccharomyces cerevisiae REV1 is required for mutagenesis induced by UV light. Proc Natl Acad Sci U S A. 2000;97(8):4186–91.
Makridakis NM, Reichardt JK. Translesion DNA polymerases and cancer. Front Genet. 2012;3:174.
Sakiyama T, Kohno T, Mimaki S, Ohta T, Yanagitani N, Sobue T, et al. Association of amino acid substitution polymorphisms in DNA repair genes TP53, POLI, REV1 and LIG4 with lung cancer risk. Int J Cancer. 2005;114(5):730–7.
He X, Ye F, Zhang J, Cheng Q, Shen J, Chen H. REV1 genetic variants associated with the risk of cervical carcinoma. Eur J Epidemiol. 2008;23(6):403–9.
Goricar K, Kovac V, Jazbec J, Zakotnik B, Lamovec J, Dolzan V. Translesion polymerase genes polymorphisms and haplotypes influence survival of osteosarcoma patients. OMICS. 2015;19(3):180–5.
Wang H, Wu W, Wang HW, Wang S, Chen Y, Zhang X, et al. Analysis of specialized DNA polymerases expression in human gliomas: association with prognostic significance. Neuro-Oncology. 2010;12(7):679–86.
Wojtaszek JL, Chatterjee N, Najeeb J, Ramos A, Lee M, Bian K, et al. A small molecule targeting mutagenic Translesion synthesis improves chemotherapy. Cell. 2019;178(1):152–9 e11.
Masumura K, Msuda S. Research on environmental mutagenesis from young scientists - the open symposium of the Japanese Environmental Mutagen Society (JEMS) in 2017. Genes Environ. 2017;39:26.
Jansen JG, Langerak P, Tsaalbi-Shtylik A, van den Berk P, Jacobs H, de Wind N. Strand-biased defect in C/G transversions in hypermutating immunoglobulin genes in Rev1-deficient mice. J Exp Med. 2006;203(2):319–23.
Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22.
Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol. 2012;12(7):517–31.
Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102(5):553–63.
Rada C, Di Noia JM, Neuberger MS. Mismatch recognition and uracil excision provide complementary paths to both Ig switching and the a/T-focused phase of somatic mutation. Mol Cell. 2004;16(2):163–71.
Krijger PH, Tsaalbi-Shtylik A, Wit N, van den Berk PC, de Wind N, Jacobs H. Rev1 is essential in generating G to C transversions downstream of the Ung2 pathway but not the Msh2+Ung2 hybrid pathway. Eur J Immunol. 2013;43(10):2765–70.
Zan H, White CA, Thomas LM, Mai T, Li G, Xu Z, et al. Rev1 recruits ung to switch regions and enhances du glycosylation for immunoglobulin class switch DNA recombination. Cell Rep. 2012;2(5):1220–32.
Jansen JG, Tsaalbi-Shtylik A, de Wind N. Roles of mutagenic translesion synthesis in mammalian genome stability, health and disease. DNA Repair (Amst). 2015;29:56–64.
Martin-Pardillos A, Tsaalbi-Shtylik A, Chen S, Lazare S, van Os RP, Dethmers-Ausema A, et al. Genomic and functional integrity of the hematopoietic system requires tolerance of oxidative DNA lesions. Blood. 2017;130(13):1523–34.
Masuda K, Ouchida R, Li Y, Gao X, Mori H, Wang JY. A critical role for REV1 in regulating the induction of C:G transitions and a:T mutations during Ig gene hypermutation. J Immunol. 2009;183(3):1846–50.
Tsaalbi-Shtylik A, Verspuy JW, Jansen JG, Rebel H, Carlee LM, van der Valk MA, et al. Error-prone translesion replication of damaged DNA suppresses skin carcinogenesis by controlling inflammatory hyperplasia. Proc Natl Acad Sci U S A. 2009;106(51):21836–41.
Sasatani M, Xi Y, Kajimura J, Kawamura T, Piao J, Masuda Y, et al. Overexpression of Rev1 promotes the development of carcinogen-induced intestinal adenomas via accumulation of point mutation and suppression of apoptosis proportionally to the Rev1 expression level. Carcinogenesis. 2017;38(5):570–8.
Toyoshima M, Honda H, Masuda Y, Kamiya K. Development of the sensitive assay system for tritium risk assessment using Rev1 transgenic mouse. Fusion Sci Technol. 2011;60:1204.
Acknowledgments
We wish to thank Dr. Kenichi Masumura and Dr. Shuichi Masuda, the organizers of the 2017 JEMS Symposium, and the Japanese Environmental Mutagen Society. We also thank the members of the Department of Experimental Oncology, Research Institute for Radiation Biology and Medicine, Hiroshima University, for helpful discussions and encouragement.
Funding
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT)/ Japan Society for the Promotion of Science (JSPS) and grants from the Ministry of Health, Labour and Welfare (to KK and MS). This work was also partially supported by NIFS Collaborative Research Program (NIFS10KOBS015), (NIFS13KOBA028), (NIFS17KOCA002), and by MEXT Funds for the Development of Human Resources in Science and Technology “The Initiative for Realizing Diversity in the Research Environment (Collaboration Type)”.
Author information
Authors and Affiliations
Contributions
MS, EZ and KK contributed to writing the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sasatani, M., Zaharieva, E.K. & Kamiya, K. The in vivo role of Rev1 in mutagenesis and carcinogenesis. Genes and Environ 42, 9 (2020). https://doi.org/10.1186/s41021-020-0148-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41021-020-0148-1