Abstract
Background
Nebulised antibiotics are frequently used for the prevention or treatment of ventilator-associated pneumonia. Many factors may influence pulmonary drug concentrations with inaccurate dosing schedules potentially leading to therapeutic failure and/or the emergence of antibiotic resistance. We describe a research pathway for studying the pharmacokinetics of a nebulised antibiotic during mechanical ventilation using in vitro methods and ovine models, using tobramycin as the study antibiotic.
Methods
In vitro studies using a laser diffractometer and a bacterial-viral filter were used to measure the effect of the type and size of tracheal tubes and antibiotic concentration on the particle size distribution of the tobramycin 400 mg (4 ml; 100 mg/ml) and 160 mg (4 ml, 40 mg/ml) aerosol and nebulised mass delivered. To compare the regional drug distribution in the lung of two routes (intravenous and nebulised) of drug administration of tobramycin 400 mg, technetium-99m-labelled tobramycin 400 mg with planar nuclear medicine imaging was used in a mechanically ventilated ovine model. To measure tobramycin concentrations by intravenous and nebulised tobramycin 400 mg (4 ml, 100 mg/ml) administration in the lung interstitial space (ISF) fluid and blood of mechanically ventilated sheep, the microdialysis technique was used over an 8-h duration.
Results
Tobramycin 100 mg/ml achieved a higher lung dose (121.3 mg) compared to 40 mg/ml (41.3 mg) solution. The imaging study with labelled tobramycin indicated that nebulised tobramycin distributed more extensively into each lung zone of the mechanically ventilated sheep than intravenous administration. A higher lung ISF peak concentration of tobramycin was observed with nebulised tobramycin (40.8 mg/l) compared to intravenous route (19.0 mg/l).
Conclusions
The research methods appear promising to describe lung pharmacokinetics for formulations intended for nebulisation during mechanical ventilation. These methods need further validation in an experimental pneumonia model to be able to contribute toward optimising dosing regimens to inform clinical trials and/or clinical use.
Similar content being viewed by others
Background
Ventilator-associated pneumonia (VAP) is common in intensive care patients and is associated with high morbidity and mortality rates [1]. To improve outcomes, nebulised antibiotic therapy has been recommended for prevention [2] and treatment of VAP [3]. However, there is controversy, with recent guidelines not recommending the use of nebulised antibiotics, citing a lack of robust effectiveness data, possibly due to inadequate trial designs and/or ineffective drug delivery [4]. Antibiotic nebulisation can potentially be a viable alternative to conventional therapy for lung infection because if administered appropriately, it should enable high lung antibiotic concentrations without significant side effects [5]. However, there are a number of factors involved in achieving optimal delivery [6, 7], which need to be characterised to ensure desired concentrations are achieved. These include physicochemical properties of the antibiotics, tracheal tube size and type [7,8,9], besides factors associated with mechanical ventilation such as ventilator settings, circuit-related factors, nebuliser factors and patient-related factors such as airway geometry and patency [7]. Due to methodological limitations, the pharmacokinetics (PK) of nebulised antibiotics during mechanical ventilation is not well understood.
For effective nebulisation therapy with mechanical ventilation, it is essential to optimise these factors. Vital information regarding drug deposition, metabolism, absorption, kinetic profile and tolerability should be considered when designing optimised dosing regimens [10]. The clinical environment provides limited opportunities to accurately study these numerous factors affecting nebulised drug delivery and lung concentration of antibiotics [10]. Hence, in vitro studies are essential to study these factors, in isolation and in combination [11]. Further to this, animal models can allow the manipulation of variables and study of their effects, which may not be feasible in human subjects [12]. Although small animals have been extensively used for these purposes [13, 14], when the study involves factors that may significantly affect drug delivery such as the airway and lung anatomy as well as circulatory parameters, large animals are preferable as they more accurately represent the human scenario [15, 16]. The pathophysiological changes that occur in respiratory diseases could influence the PK of nebulised antibiotics [7]. Sheep are considered an appropriate large animal model to study pulmonary PK associated with different devices and/or formulations [10] in healthy and in experimental pneumonia models [17, 18]. In this paper, we describe an approach to providing a detailed characterisation of pulmonary PK of nebulised drug delivery using a combination of in vitro and ovine studies.
The specific aims of this research are:
-
1.
To calibrate the nebulised delivery system during mechanical ventilation.
-
2.
To quantify and compare the regional pulmonary distribution when administered via intravenous and nebulised routes during mechanical ventilation in a healthy ovine model.
-
3.
To calibrate the microdialysis system and its application to quantify and compare lung interstitial fluid (ISF) concentrations of tobramycin when administered via intravenous and nebulised routes during mechanical ventilation in a healthy ovine model.
Methods
The program of research comprises of complementary studies investigating different aspects of nebulised antibiotic therapy with a view to addressing key questions (Table 1). Each individual sub-study is described in detail below.
Calibrating the nebulised delivery system during mechanical ventilation
Two experimental techniques were employed for this purpose.
Aerosol particle size distribution
Equipment
We used an adult-use mechanical ventilator and circuits (Puritan Bennett 840 ventilator, Covidien-Medtronic, Mansfield, MA) in conjunction with a disposable vibrating mesh nebuliser (VMN) (Aeroneb pro, Aerogen, Inc., Galway, Ireland) placed in the inspiratory limb of the circuit as per the recommendations [19]. Although there are a number of delivery devices available, VMN is recommended for nebulised antibiotic therapy in mechanically ventilated patients [19, 20]. We investigated the effects of different concentrations of tobramycin (Tobra-day, Phebra) 100 mg/ml and 40 mg/ml (Tobramycin PF, Pfizer, Australia) on the nebulisation characteristics. We also investigated the effects of different types of tracheal tubes (endotracheal tube vs tracheostomy tube; Portex, Smiths Medical International Ltd., UK) with tube size 9.0 mm internal diameter.
Study method
As ventilator settings can affect aerosol drug delivery [7], the parameters set here were adjusted for their use in the ovine in vivo studies later. Ventilator parameters were fixed for all the in vitro experiments with a tidal volume of 500 ml, respiratory rate 14/min, square wave flow pattern, flow rate 40 lpm, positive end-expiratory pressure (PEEP) = 5 and FiO2 = 0.21.
As recommended by expert consensus, humidification was turned off for the experiments [21]. The tips of the tracheal tubes were placed in the receptacle of the laser diffractometer (Spraytec, Malvern Instruments, UK) using a silicone adaptor. The principle of the laser diffraction technique is shown in Fig. 1. The circuit was closed using an adult test lung (Dräger Medical, Lubeck, Germany) thus enabling continuous operation. The nebuliser was then filled with 4 ml tobramycin 100 mg/ml (400 mg) and then repeated with 4 ml of 40 mg/ml concentration (160 mg). Nebulisation was considered to be complete when no mist was visible from the nebuliser. The nebuliser and bacterial filter were changed after every experiment as per the guidelines [19]. Figure 2a demonstrates the study set-up in the laboratory.
Data collection and analysis
The following data were collected: baseline temperature, pH and viscosity of the tobramycin solution and nebulisation time. The viscosity of the tobramycin solution was determined at a constant temperature of 25 °C using a DHR-3 Rheometer (TA Instruments, USA). Particle size distributions were expressed as dv10 (volume diameter under which 10% of the sample resides), dv50 (volume median diameter) and dv90 (volume diameter under which 90% of the sample resides). In addition to dv10, dv50 and dv90, the percentages of particles between sizes 1 and 5 μm (the sizes conventionally considered for lung deposition) obtained from the laser diffractometer were included in this work.
In vitro nebulised mass study
Equipment
Adult-use mechanical ventilator, VMN, endotracheal tubes and tracheostomy tubes and tobramycin were used as per the particle size distribution study described above. In addition, respiratory (bacterial and viral) filters (filter pads, Fisher and Paykel, Auckland, New Zealand) were used.
Study method
Using a previously described methodology [22], we used the same set-up as described previously and insert the tip of the tracheal tube into a respiratory (bacterial and viral) filter (filter pads, Fisher and Paykel, Auckland, New Zealand). The circuit was closed using an adult test lung (Dräger Medical, Lubeck, Germany) thus allowing continuous operation. The nebuliser was filled with 4 ml tobramycin 100 mg/ml (400 mg) instilled with the nebulisation process to continue until there is no visible mist from the nebuliser. The ventilator was paused whilst the filter was removed from the circuit and stored at − 80 °C for drug assay using liquid chromatography with tandem mass spectrometry (LC/MS-MS). The process was repeated with 4 ml tobramycin 40 mg/ml (160 mg). The nebuliser was weighed before and after nebulisation to describe residual drug quantity. The experiment was done with each of the tracheal tubes as previously mentioned. Each experiment was repeated in triplicate. The nebuliser and bacterial filter were changed after every experiment as per the guidelines [19]. The study set-up was as shown in Fig. 2b.
Sample and data analysis
Tobramycin was extracted from the filter using water in a sonication bath, stored at − 80 °C and analysed later using liquid chromatography-tandem mass spectrometry (LC-MS/MS) as described later. The amount of drug was reported as milligrams (mg) of tobramycin. The percentage of the nebulised drug on the filter was calculated and expressed with ranges. Combined data from the particle sizing and nebulised mass study was used to describe the lung dose, i.e. the amount of drug in the effective particle size range for distal lung deposition.
Development of an ovine model for aerosol studies
Design
We used a healthy ovine model to assess the regional drug deposition and lung ISF concentrations of intravenously and nebulised tobramycin.
Ethics
All the ovine studies have been ethically approved by the Queensland University of Technology animal ethics committee approval (Approval no. 1100000052; lung ISF study) and University of Queensland animal ethics committee approval (Approval no. SOM/268/16/RBWH; regional distribution studies).
Operating room set up
The basic operating room set-up and the anaesthesia and instrumentation components of the study were the same for the regional drug distribution study and the tobramycin concentration in the lung ISF study.
The operating room was equipped to manage and monitor for the animals’ health and well-being. A ‘head box’ or suspender was used to stabilise and immobilise the head whilst being sedated and mechanically ventilated in a prone position. Syringe drivers and infusion pumps were used for fluid infusions and anaesthesia maintenance. Precision microdialysis pumps (CMA 107, CMA microdialysis, Solna, Sweden) were used for microdialysis. A fibreoptic bronchoscope ((Pentax FB15TV, Philips, Tokyo, Japan) was used to obtain mini-bronchoalveolar lavage (BAL) samples. A qualified vet supervised the procedure at all times.
Anaesthesia and instrumentation
Pre-study
The veterinary specialist examined all Merino sheeps, and baseline blood samples were collected with only healthy Merino sheep weighing 40 to 50 kg selected for the study. The sheeps were fasted overnight and were led to the operating room in a protective sling with face, neck and chest area shaved.
Venous access, anaesthesia induction, airway access and mechanical ventilation
Using aseptic technique, a central venous line was inserted through the right jugular vein under local anaesthesia. Anaesthesia was commenced using induction agents, midazolam 0.5 mg/kg and alfaxalone 3 mg/kg (Jurox Pty Ltd., Hunter Valley, Australia). Orotracheal intubation was performed using an endotracheal tube (Portex, Smiths Medical, London, UK). Tracheal intubation was confirmed using a colorimeter and mechanical ventilation commenced. Ventilator parameters used throughout the experiment fulfilled the Acute Respiratory Distress Syndrome Network criteria for lung protective ventilation [23]. Initial ventilator settings for all the sheeps were respiratory rate 14 breaths per minute, tidal volume 500 ml, PEEP 5 cm H2O and fraction of inspired oxygen (FiO2) 0.21. During nebulisation, square wave flow pattern was applied, and the humidifier was turned off. Respiratory rate and FiO2 were adjusted according to arterial blood gas (ABG) results to maintain normal parameters. Due to the long length of the sheep airway, it was necessary to perform a tracheostomy to represent the human airway. This was performed with an aseptic technique using a size 9 tracheostomy tube (Portex, Smiths Medical, London, UK) and confirmed using bronchoscope and capnography. The airway was assessed using bronchoscopy for any anatomical variation or pathological abnormality. The animal was subsequently positioned in a prone position. Anaesthesia was maintained using ketamine 3–5 mg/kg/h, midazolam 0.25–0.5 mg/kg/h and alfaxalone 4–6 mg/kg/h infusion titrated to effect. Hydration was maintained using Hartmann’s solution at the rate of 2 ml/kg. The animal was kept warm using warming blankets. The operating room set-up, anaesthesia and instrumentation were common to the two ovine studies—regional drug distribution and microdialysis.
Monitoring
The sheep had routine monitoring using ECG, arterial blood pressure (ABP), end-tidal carbon dioxide monitor (ETCO2), pulse oximetry and central venous pressure (CVP) monitoring. An invasive arterial line was inserted in the facial artery using a cut-down method for the ABP monitoring and ABG sampling. Vital physiological data was recorded using Marquette Solar 8000 monitor (GE Healthcare, Little Chalfont, UK). A urinary catheter and orogastric tube were also inserted. Regular ABGs (two hourly) were obtained to confirm the adequacy of ventilation and other parameters such as electrolytes, glucose and lactate levels.
Comparing regional pulmonary distribution of intravenous and nebulised tobramycin during mechanical ventilation
Computed tomography scan
Following adequate instrumentation, a CT scan of the chest was performed to confirm normal lung anatomy, and the images were used to obtain a region of interest (ROI) during image analysis. The ROI provides the lung outline for superimposition onto the gamma camera images for subsequent analysis.
Drug administration
Nebulisation
Using a disposable (VMN) (Aeroneb pro, Aerogen, Inc., Galway, Ireland) in the inspiratory limb of the ventilator circuit, tobramycin 400 mg (100 mg/ml) labelled with technetium-99m was nebulised until no further mist is visible. Previous studies have shown that the aerosol characteristics of labelled tobramycin are similar to that of unlabelled tobramycin [24].
Intravenous
Technetium-99m-labelled tobramycin 400 mg was injected via a central venous line as a 30-min infusion. The lumen was flushed with 100 ml of 0.9% saline.
Image acquisition
A single-head gamma camera (GE Starcam 600 XR/T Gamma Camera, GE Healthcare Global Diagnostic Imaging, Waukesha, WI, USA) was used to acquire planar images. The CT scan and the gamma camera scan equipment were located within the same research facility. Dynamic (10 s frames) and static scans were obtained. Images were acquired from lateral, dorsal and ventral aspects. Blood samples at predetermined intervals were obtained to coincide with the scans. Radiation count in the nebuliser and the syringe was performed before and after the nebulisation and intravenous administration, respectively, to define the dose and radioactivity administered.
Euthanasia and tissue sampling
At the completion of the study, the sheep was euthanized using sodium pentobarbitone (295 mg/ml, 0.5 ml/kg). Death was confirmed by loss of cardiac electrical activity and ABP trace. After euthanasia, the organs were surgically retrieved. Direct tissue sampling from pre-defined regions of the lungs, liver, both kidneys and urine was performed. The remains of the animals were frozen and stored until disposed of via incineration.
Data analysis
The images were analysed and reported as radioactivity distribution into peripheral to central ratio (P/C ratio), also known as ‘penetration index’ for the nebulised tobramycin group. For this analysis, the central region represents the conducting airways, and the peripheral represents the alveoli. Parameters used to compare the intravenous and nebulised route of administration include dorsal to ventral ratio, right to left lung ratio and upper/middle/lower lung zone distribution. The quantitative analysis included describing the radioactivity in per gram of pulmonary and extra-pulmonary tissue. Blood radioactivity was compared between the two groups as a surrogate measure of drug level in the systemic circulation.
Calibration of the microdialysis system using in vitro microdialysis recovery study
Equipment
The study used fresh frozen plasma and a magnetic stirrer CMA 63 microdialysis catheters (molecular weight cut-off of 20 kDa; an outer diameter of 0.6 mm and a membrane length of 30 mm; CMA Microdialysis AB, Stockholm, Sweden), CMA 107 precision microinfusion pump (CMA Microdialysis AB, Stockholm, Sweden) and Cole-Parmer two-syringe infusion pump 230 VAC CE (John Morris Group, Chatswood, Australia).
Study method
Microdialysis probes were perfused with Ringer’s solution at three different flow rates 1, 1.5 and 2 μl/min using the precision pump. The membranes of the microdialysis probes were immersed in 50 ml solution of 5 μg/mL of tobramycin and 5 μg/mL vancomycin concentrations. The samples were collected at 20-min intervals for 100 min with experiments repeated in triplicate.
Sample analysis
Samples were stored at − 80 °C and assayed using LC-MS/MS methods described previously.
Recovery rates were expressed as relative recovery (RR):
where Cdialysate = concentration of antibiotic in the microdialysate and Csolution = concentration of antibiotic in the test solution.
Figure 3a, b describes the principles of microdialysis when used for the study of inhaled antibiotics and intravenous antibiotics, respectively. The principles have been described in detail previously [25].
Schematic diagram demonstrating the principle of lung microdialysis. The unidirectional perfusate flow provides a concentration gradient for diffusion of analytes across a semi-permeable membrane placed in the lung interstitium. The dialysate is then collected in microvials for analysis. a Principles of lung microdialysis with nebulised antibiotics—showing higher antibiotic concentration in the alveoli. b Principles of lung microdialysis with intravenous antibiotics—showing higher antibiotic concentration in the pulmonary arteriole
Comparing lung ISF concentrations of intravenous and nebulised tobramycin
Microdialysis catheter priming and set-up
Various factors influence in vivo recovery of analytes; hence, in addition to in vitro studies, it is essential to perform in vivo recovery [25]. Methods for in vivo recovery calculations have been described in the literature [26]. Internal indicator technique was used in this study. The perfusate for microdialysis was prepared using 0.1 ml (4 mg) of gentamicin 80 mg/2 ml vial. This was injected into a 1-l bag of Hartmann’s solution to make up a concentration of 4 μg/ml which was then be used as the perfusate for the catheters. Gentamicin was used as an internal standard to enable in vivo recovery calculations and was chosen due to similarities with tobramycin (molecular weight of gentamicin = 467 vs tobramycin = 477 g/mol). Using CMA 107 battery pumps, the microdialysis catheters were primed with the perfusate solution at a rate of 5 μl/min for 10 min. The catheters were then inserted, and the perfusate rate was reduced to 1 μl/min for the remainder of the experiment. Once inserted, the catheters were perfused for a period of 1 h to achieve a steady state equilibrium.
Insertion of microdialysis catheters
After anaesthesia induction and instrumentation, bilateral thoracotomies were performed in the fifth intercostal space exposing the interlobar fissures. A CMA 63 microdialysis catheter (CMA Microdialysis AB, Stockholm, Sweden) with a molecular weight cut-off of 20 kDa, an outer diameter of 0.6 mm and a membrane length of 30 mm was used. Four microdialysis catheters were inserted, in the right upper and lower and the left upper and lower lobes using an introducer needle under direct vision. Bioglue (Coseal surgical sealant, Cohesion technologies, US) was applied at the lung puncture site to minimise catheter movement post-insertion. An intercostal catheter was inserted in each of the pleural spaces and low-pressure suction applied, and the thoracotomy incisions will be closed. An intravascular microdialysis catheter, CMA 64 (CMA Microdialysis AB, Stockholm, Sweden) with a molecular weight cut-off 20 kDa and a membrane length of 20 mm, was inserted in the left jugular vein through a percutaneously inserted 18G cannula (B. Braun Australia Pty Ltd., Bella Vista, Australia).
Drug administration
Nebulisation
Using a disposable (VMN) (Aeroneb pro, Aerogen, Inc., Galway, Ireland) in the inspiratory limb of the ventilator circuit, tobramycin 400 mg (Tobra-day, Phebra 500 mg/5 ml) was nebulised. The nebulisation was continued until the visible mist from the nebuliser stopped. A baseline pre-nebulisation microdialysis sample was collected (T0).
Intravenous antibiotic administration
Tobramycin 400 mg (Tobra-day, Phebra 500 mg/5 ml) was administered intravenously through the central venous line as a 30-min infusion. A baseline pre-intravenous administration, microdialysis sample was collected (T0).
Following the drug administration (nebulised and intravenous), the samples were collected at every 20 min in a microvial (CMA, Microdialysis AB) for 8 h. The samples were stored at − 80 °C for further analysis.
Bronchoalveolar lavage
A mini-BAL was performed at T = 60 min, 4 h and 7 h using two 20 ml aliquots of normal saline as previously described [27, 28]. This procedure was performed using a fibreoptic bronchoscope with the tip wedged in the right lower lobe and left lower lobe bronchi. The actual epithelial lining fluid (ELF) concentrations of tobramycin were obtained from the measured BAL fluid concentrations after correction for dilution according to the equation:
where CBAL corresponds to the tobramycin concentrations measured in the BAL fluid, and UreaBAL and UreaPLASMA correspond to the concentrations of urea determined in BAL fluid and plasma, respectively. Blood samples were taken for measuring urea to assess the adequacy of BAL.
Euthanasia, catheter inspection and organ retrieval
At the completion of the study, the sheeps were humanely euthanized using sodium pentobarbitone (295 mg/ml, dose 0.5 ml/kg). Using chest retractors, the microdialysis catheters were exposed and inspected to confirm that the catheters remained inside the lungs throughout the procedure. The catheters were inspected for obvious evidence of damage. The lungs were retrieved, and the sections of bilateral upper and lower lobes were visually inspected and harvested for further drug concentration analysis. The remains of the animals were frozen and stored until disposal via incineration.
LC-MS/MS method for sample analysis
The tobramycin concentrations in microdialysis samples reported were measured by an LC-MS/MS method using a simple dilution preparation and HILIC chromatography on a Shimadzu Nexera2 system coupled to a Shimadzu 8030+ triple quadrupole mass spectrometer (Kyoto, Japan). The assay method has validation for linearity (quadratic line from 0.1 to 20 μg/ml with precision and accuracy of all calibration standards within 8%), LLOQ (precision and accuracy within 6% at 0.1 μg/mL), matrix effects (normalised matrix factor precision within 9% at 0.2 and 16 μg/ml) and precision and accuracy (precision and accuracy within 10% at 0.3, 2 and 16 μg/ml) using the FDA criteria for bioanalysis [29]. Recovery data was used to calculate the corrected tobramycin concentrations in the microdialysate.
Statistical considerations
The results were expressed as mean, median, minimum, maximum and standard deviation. For the in vitro particle size and inhaled mass study, multiple linear regression was used to estimate the effect of the variables on the outcomes. For the in vitro microdialysis recovery studies, a linear regression model using recovery as the dependent variable and flow rate as the independent variable was applied. Similarly, the blood radioactivity levels in the ovine study were compared using Bayesian regression analysis using a random intercept for each sheep and Bayesian p values were obtained. These analyses were conducted using R version 3.4.2 and WinBUGS version 1.4.3 for the Bayesian regression analysis. The individual studies were not powered to detect statistical significance, and instead, we used confidence intervals where appropriate and discuss the size of the differences in terms of their clinical as well as statistical significance. The plots of summary statistics were used to compare groups and results over time.
Results
The simulated adult mechanical ventilation model to evaluate the particle size distribution and quantify the efficacy of the aerosol system showed that fine particle fraction (1 to 5 μm) was significantly higher for 100 mg/ml solution compared to 40 mg/ml solution (99.4 ± 0.3% vs 82.3 ± 6%; p < 0.001). The lung dose delivered was significantly higher for 100 mg/ml solution compared to 40 mg/ml solution (132.7 ± 12.6 mg vs 39.2 ± 1.1 mg; p < 0.001). Nebulisation duration was significantly higher for the 100 mg/ml solution compared to 40 mg/ml solution for endotracheal tube (18.56 ± 2.36 vs 7.88 ± 1.08 min; p < 0.001) and tracheostomy tube (14.63 ± 1.14 vs 12 ± 1.4 min; p < 0.001). Tracheal tube type had a minimal effect which was not statistically significant, on the aerosol delivery parameters. Other parameters like dv10 and dv95 were not statistically different. Table 2 summarises the differences between the two formulations. The experiments were performed at a temperature of 25 °C.
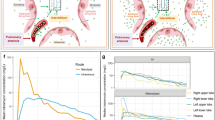
The ovine model for the comparative regional lung distribution study between aerosolized and intravenous tobramycin was successfully established. Female sheeps (ewes, n = 4) were used for the study. There were no adverse events documented during the ovine studies. For the nebulised sheep, the P/C ratio (penetration index) was 0.9 for the right lung and 0.87 for the left lung. The blood radioactivity was significantly higher for the sheep with intravenous tobramycin compared to that for the nebulised tobramycin (209.2 ± 97.6 Mbq/ml vs 33.3 ± 5.4 Mbq/ml; p < 0.001). The right to left ratio was 0.9 for intravenous and 0.89 for the nebulised sheep. Figure 4 shows the comparative gamma camera scan images between i.v. and nebulised technetium-labelled tobramycin. Table 3 shows that the liver, kidney and urine levels were lower for nebulised compared to intravenous sheep.
Microdialysis recovery in the simulated in vivo conditions showed that relative recovery for vancomycin was 32.6 ± 0.09%, 27.5 ± 0.09% and 20.7 ± 0.04% for perfusate flow rates of 1, 1.5 and 2 μl/min, respectively. Thus, for vancomycin, the highest relative recovery was for a perfusate flow rate of 1 μl/min (95% CI 24.8 to 35.7%). For tobramycin, the recoveries were 69 ± 3.74%, 69.5 ± 3.47% and 65 ± 3.8%, respectively. The differences were not seen to be statistically significant.
The lung isf concentrations in the mechanically ventilated ovine model showed that area under the concentration- time curve (mean) and peak (or maximum) concentration in the concentration- time curve (mean) for nebulized tobramycin was 313 mg-h/liter and 535.8 mg/liter and for intravenous tobramycin the values were 90.82 mg-h/liter and 22.2 mg/liter respectively, for intravenous tobramycin. Figure 5 shows lung ISF concentrations with nebulised tobramycin compared to intravenous tobramycin. For the nebulised tobramycin sheep, ELF concentration was 1.94 mg/l at 1 h and for intravenous tobramycin sheep, and the level was 0.19 mg/l. (Table 4).
Technically the ovine model set up for the regional lung distribution and lung microdialysis study was uneventful. There were no adverse events affecting the interpretation of results. We have optimised anaesthesia, ventilation, surgical instrumentation, scintigraphy protocols, sample collection and analyses in this model.
Discussion
This paper describes an incremental model of the study of the PK of nebulised antibiotics. As the lung is a heterogeneous organ, PK studies to accurately characterise drug concentrations in the various compartments are difficult. We have used a methodological approach for the assessment of the PK of nebulised antibiotics using in vitro and ovine models. For nebulised antibiotic administration, important PK parameters are pulmonary deposition efficiency, regional distribution, pulmonary absorption and residence time [6]. For therapeutic response, the dose deposited at the site of infection in the lung is important [30] particularly the unbound concentration of the drug [6]. We studied these essential aspects of nebulised antibiotic therapy using tobramycin.
For higher concentration tobramycin (100 mg/ml), the aerosol delivery system can deliver a higher lung dose. This is important for tobramycin and other aminoglycosides that rely on peak (or maximum) concentration as the PK measure for drug effect. Aerosol particle size and the aerosolized drug mass are important parameters for assessing the efficacy of an aerosol delivery system [7]. Methods for the study of the aerosol particle size distribution and nebulised mass have been summarised previously [22, 31]. We used the laser diffractometer which is a validated technique for the assessment of aerosol particle size distribution [32] and an established technique for the nebulised mass study [22]. Recent recommendations have been to place the VMN 10–15 cm from the Y-piece [33]. The VMN was placed just before the Y-piece in the inspiratory limb to mimic local and international practices [34] as well as the manufacturer’s recommendations.
Our results suggest that mechanically ventilated sheep could be effectively used for the study of regional lung distribution studies especially for drugs administered for local effect on the lung diseases, e.g. infections. The distribution parameters of nebulised tobramycin would support its use in lung infections especially with minimal systemic and extra-pulmonary tissue levels as seen in our study. Methods of assessing pulmonary drug distribution have been summarised in detail previously [35]. Whilst single-photon emission computed tomography (SPECT) and positron emission tomography (PET) provide a 3D description of the regional drug distribution in the lungs, for most purposes, planar gamma scintigraphy is sufficient [36, 37] and feasible; therefore, we used the gamma scintigraphy method for our study.
The results of the present microdialysis calibration study suggest that large molecules like vancomycin have significantly reduced recovery and protein binding adversely affected the recovery. Perfusate flow rates also affected relative recovery rates for tobramycin and vancomycin. Thus, microdialysis-based studies for large molecules as well as highly protein-bound substances should be evaluated in vitro in a similar model. By calibrating the microdialysis system, thus depending on the study drug, it will enable the correct combination of microdialysis catheters and perfusate flow rates. The techniques used in other studies to assess pulmonary drug concentrations used could not inform adequately the longitudinal data desired over time. These studies have been previously summarised [25], but most studies employed antibiotic BAL concentrations as a surrogate [38, 39]. Some authors have used blood concentrations to perform population PK compartmental modelling [40]. For most bacterial infections, the antibiotic site of action is the ISF [41]. Lung microdialysis is an established technique to sample lung ISF for the purposes of measuring antibiotic concentrations [25]. The details of the microdialysis technique and relevant considerations have been described elsewhere [42,43,44,45]. Recovery studies are important for the accurate calculation of the antibiotic concentration in the microdialysis sample [26].
For tobramycin, the high lung ISF concentration achieved with nebulised route meets the PK requirements for drug effect. Study of drugs with different PK parameters would elucidate if the nebulised route is appropriate for therapy. Preclinical models for the study of pulmonary drug delivery have been well described [10]. Methods such as in vitro cell culture whilst providing a consistent method of assessing nebulised drugs cannot predict the pulmonary bioavailability of the drug as it cannot assess the other barriers to absorption [10]. In vitro cell culture method remains a viable alternative to test for drug toxicity. Isolated perfused lung model provides an ex vivo model for drug PK assessment but lack of tracheobronchial circulation could be a drawback in the assessment of systemic absorption [10]. In vivo models provide the most thorough quantitative and qualitative data to inform the PK of pulmonary drug delivery [46].
As airway size and type were considered an important variable in drug deposition, the in vitro aerosol characteristics were investigated with the size 9.0 mm I.D. tracheal tubes as these were the tracheal tube sizes used for the in vivo studies in sheep, thus minimising the variables in the study data.
Sheep are the ideal large animal model to study the PKs of nebulised antibiotics [12]. Their size and lung anatomical-physiological features are similar to humans [47]. By replicating the study, data could be acquired to describe the PK profile of a wide range of nebulised antibiotics. This adds valuable information for potential efficacy and toxicity of drugs and formulations. Our model can be applied to other potentially important questions and test other factors such as variations in ventilator parameters, the distribution characteristics of newer nebulised drug formulations, novel delivery devices and different patient parameters such as posture that might affect pulmonary drug delivery. There is potential for the study of other nebulised drugs where lung ISF concentrations are important, e.g. other anti-infectives like antiviral and antifungal agents, chemotherapeutic agents and immunomodulatory drugs.
Future work will consist of pharmacodynamic studies in a variety of clinically important experimental pneumonia settings. This data could be used to improve the precision and accuracy of nebulised antibiotic dosing schedules in a variety of lung infections in the pre-clinical models. The complexity of the pathophysiological changes associated with pneumonia makes the development and management of the animal models of experimental pneumonia challenging. Factors that need consideration include ethics, selection of appropriate animal species, number of subjects, inoculation routes and sampling issues, and the present animal model used account for some, but not all, of these. Clinical trials using accurate information from these mechanistic and preclinical studies could yield better therapeutic outcomes.
Conclusions
This program of research aims to apply in vitro and ovine models for the study of nebulised antibiotic delivery and lung PK. This model can be used for further studies involving drugs where knowledge of pulmonary drug concentration is essential. Systematic investigation of other aspects of nebulised antibiotic therapy using this model will introduce further refinements in nebulised antibiotic therapy thus improving patient care and outcomes.
Abbreviations
- ABG:
-
Arterial blood gas
- ABP:
-
Arterial blood pressure
- BAL:
-
Bronchoalveolar lavage
- C/P:
-
Central to peripheral ratio
- CT:
-
Computed tomography
- CVP:
-
Central venous pressure
- ELF:
-
Epithelial lining fluid
- ETCO2 :
-
End-tidal carbon dioxide
- ISF:
-
Interstitial fluid
- LC-MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- PD:
-
Pharmacodynamics
- PEEP:
-
Positive end-expiratory pressure
- PET:
-
Positron emission tomography
- PK:
-
Pharmacokinetics
- ROI:
-
Region of interest
- RR:
-
Relative recovery
- SPECT:
-
Single-photon emission computed tomography
- VAP:
-
Ventilator-associated pneumonia
- VMN:
-
Vibrating mesh nebuliser
References
Kalanuria AA, Ziai W, Mirski M (2014) Ventilator-associated pneumonia in the ICU. Crit Care 18(2):208. https://doi.org/10.1186/cc13775
Povoa FCC, Cardinal-Fernandez P, Maia IS, Reboredo MM, Pinheiro BV (2017) Effect of antibiotics administered via the respiratory tract in the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. J Crit Care 43:240–245. https://doi.org/10.1016/j.jcrc.2017.09.019
Zampieri FG, Nassar AP Jr, Gusmao-Flores D, Taniguchi LU, Torres A, Ranzani OT (2015) Nebulized antibiotics for ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care 19:150. https://doi.org/10.1186/s13054-015-0868-y
Rello J, Sole-Lleonart C, Rouby JJ, Chastre J, Blot S, Poulakou G, Luyt CE, Riera J, Palmer LB, Pereira JM, Felton T, Dhanani J, Bassetti M, Welte T, Roberts JA (2017) Use of nebulized antimicrobials for the treatment of respiratory infections in invasively mechanically ventilated adults: a position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin Microbiol Infect 23(9):629–639. https://doi.org/10.1016/j.cmi.2017.04.011
Patton JS, Byron PR (2007) Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov 6(1):67–74. https://doi.org/10.1038/nrd2153
Tayab ZR, Hochhaus G (2005) Pharmacokinetic/pharmacodynamic evaluation of inhalation drugs: application to targeted pulmonary delivery systems. Expert Opin Drug Deliv 2(3):519–532. https://doi.org/10.1517/17425247.2.3.519
Dhanani J, Fraser JF, Chan HK, Rello J, Cohen J, Roberts JA (2016) Fundamentals of aerosol therapy in critical care. Crit Care 20(1):269. https://doi.org/10.1186/s13054-016-1448-5
Dugernier J, Ehrmann S, Sottiaux T, Roeseler J, Wittebole X, Dugernier T, Jamar F, Laterre PF, Reychler G (2017) Aerosol delivery during invasive mechanical ventilation: a systematic review. Crit Care 21(1):264. https://doi.org/10.1186/s13054-017-1844-5
Ghazanfari T, Elhissi AM, Ding Z, Taylor KM (2007) The influence of fluid physicochemical properties on vibrating-mesh nebulization. Int J Pharm 339(1–2):103–111. https://doi.org/10.1016/j.ijpharm.2007.02.035
Fernandes CA, Vanbever R (2009) Preclinical models for pulmonary drug delivery. Expert Opin Drug Deliv 6(11):1231–1245. https://doi.org/10.1517/17425240903241788
Jaafar-Maalej C, Andrieu V, Elaissari A, Fessi H (2009) Assessment methods of inhaled aerosols: technical aspects and applications. Expert Opin Drug Deliv 6(9):941–959. https://doi.org/10.1517/17425240903117244
Phalen RF, Oldham MJ, Wolff RK (2008) The relevance of animal models for aerosol studies. J Aerosol Med Pulm Drug Deliv 21(1):113–124. https://doi.org/10.1089/jamp.2007.0673
Mercier E, Darrouzain F, Montharu J, Guillon A, Diot P, Paintaud G, Vecellio L (2014) Lung and serum teicoplanin concentration after aerosol and intravenous administration in a rat model. J Aerosol Med Pulm Drug Deliv 27(4):306–312. https://doi.org/10.1089/jamp.2013.1060
MacLoughlin RJ, Higgins BD, Laffey JG, O’Brien T (2009) Optimized aerosol delivery to a mechanically ventilated rodent. J Aerosol Med Pulm Drug Deliv 22(4):323–332. https://doi.org/10.1089/jamp.2008.0717
Cryan SA, Sivadas N, Garcia-Contreras L (2007) In vivo animal models for drug delivery across the lung mucosal barrier. Adv Drug Deliv Rev 59(11):1133–1151. https://doi.org/10.1016/j.addr.2007.08.023
Sakagami M (2006) In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev 58(9–10):1030–1060. https://doi.org/10.1016/j.addr.2006.07.012
Lange M, Hamahata A, Enkhbaatar P, Esechie A, Connelly R, Nakano Y, Jonkam C, Cox RA, Traber LD, Herndon DN, Traber DL (2008) Assessment of vascular permeability in an ovine model of acute lung injury and pneumonia-induced Pseudomonas aeruginosa sepsis. Crit Care Med 36(4):1284–1289. https://doi.org/10.1097/CCM.0b013e318169ef74
Enkhbaatar P, Joncam C, Traber L, Nakano Y, Wang J, Lange M, Connelly R, Kulp G, Saunders F, Huda R, Cox R, Schmalstieg F, Herndon D, Traber D (2008) Novel ovine model of methicillin-resistant Staphylococcus aureus-induced pneumonia and sepsis. Shock 29(5):642–649. https://doi.org/10.1097/shk.0b013e318158125b
Rello J, Sole-Lleonart C, Rouby JJ, Chastre J, Blot S, Poulakou G, Luyt CE, Riera J, Palmer LB, Pereira JM, Felton T, Dhanani J, Bassetti M, Welte T, Roberts JA (2017) Use of nebulized antimicrobials for the treatment of respiratory infections in invasively mechanically ventilated adults: a position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2017.04.011
Poulakou G, Siakallis G, Tsiodras S, Arfaras-Melainis A, Dimopoulos G (2017) Nebulized antibiotics in mechanically ventilated patients: roadmap and challenges. Expert Rev Anti-Infect Ther 15(3):211–229. https://doi.org/10.1080/14787210.2017.1268052
Rello J, Rouby JJ, Sole-Lleonart C, Chastre J, Blot S, Luyt CE, Riera J, Vos MC, Monsel A, Dhanani J, Roberts JA (2017) Key conceptional considerations on nebulization of antimicrobial agents to mechanically ventilated patients. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2017.03.018
O’Riordan TG, Palmer LB, Smaldone GC (1994) Aerosol deposition in mechanically ventilated patients. Optimizing nebulizer delivery. Am J Respir Crit Care Med 149(1):214–219. https://doi.org/10.1164/ajrccm.149.1.8111585
Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342(18):1301–1308. https://doi.org/10.1056/NEJM200005043421801
Mukhopadhyay S, Staddon GE, Eastman C, Palmer M, Davies ER, Carswell F (1994) The quantitative distribution of nebulized antibiotic in the lung in cystic fibrosis. Respir Med 88(3):203–211
Dhanani J, Roberts JA, Chew M, Lipman J, Boots RJ, Paterson DL, Fraser JF (2010) Antimicrobial chemotherapy and lung microdialysis: a review. Int J Antimicrob Agents 36(6):491–500. https://doi.org/10.1016/j.ijantimicag.2010.08.013
de Lange ECM (2013) Recovery and calibration techniques: toward quantitative microdialysis. In: Müller M (ed) Microdialysis in drug development. Springer New York, New York, NY, pp 13–33. https://doi.org/10.1007/978-1-4614-4815-0_2
Baldwin DR, Wise R, Andrews JM, Honeybourne D (1991) Microlavage: a technique for determining the volume of epithelial lining fluid. Thorax 46(9):658–662
Boselli E, Breilh D, Duflo F, Saux MC, Debon R, Chassard D, Allaouchiche B (2003) Steady-state plasma and intrapulmonary concentrations of cefepime administered in continuous infusion in critically ill patients with severe nosocomial pneumonia. Crit Care Med 31(8):2102–2106. https://doi.org/10.1097/01.CCM.0000069734.38738.C8
FDA (2013) Guidance for industry: bioanalytical method validation. Draft Guidance
Pritchard JN (2001) The influence of lung deposition on clinical response. J Aerosol Med 14(Suppl 1):S19–S26. https://doi.org/10.1089/08942680150506303
Shekunov BY, Chattopadhyay P, Tong HH, Chow AH (2007) Particle size analysis in pharmaceutics: principles, methods and applications. Pharm Res 24(2):203–227. https://doi.org/10.1007/s11095-006-9146-7
Vecellio None L, Grimbert D, Becquemin MH, Boissinot E, Le Pape A, Lemarie E, Diot P (2001) Validation of laser diffraction method as a substitute for cascade impaction in the European Project for a Nebulizer Standard. J Aerosol Med 14(1):107–114. https://doi.org/10.1089/08942680152007954
Rello J, Rouby JJ, Sole-Lleonart C, Chastre J, Blot S, Luyt CE, Riera J, Vos MC, Monsel A, Dhanani J, Roberts JA (2017) Key considerations on nebulization of antimicrobial agents to mechanically ventilated patients. Clin Microbiol Infect 23(9):640–646. https://doi.org/10.1016/j.cmi.2017.03.018
Ehrmann S, Roche-Campo F, Bodet-Contentin L, Razazi K, Dugernier J, Trenado-Alvarez J, Donzeau A, Vermeulen F, Thevoz D, Papanikolaou M, Edelson A, Yoshido HL, Piquilloud L, Lakhal K, Lopes C, Vicent C, Desachy A, Apiou-Sbirlea G, Isabey D, Brochard L, Reva Research N, Group AIS (2016) Aerosol therapy in intensive and intermediate care units: prospective observation of 2808 critically ill patients. Intensive Care Med 42(2):192–201. https://doi.org/10.1007/s00134-015-4114-5
Scheuch G, Bennett W, Borgstrom L, Clark A, Dalby R, Dolovich M, Fleming J, Gehr P, Gonda I, O’Callaghan C, Taylor G, Newman S (2010) Deposition, imaging, and clearance: what remains to be done? J Aerosol Med Pulm Drug Deliv 23(Suppl 2):S39–S57. https://doi.org/10.1089/jamp.2010.0839
Newman S, Fleming J (2011) Challenges in assessing regional distribution of inhaled drug in the human lungs. Expert Opin Drug Deliv 8(7):841–855. https://doi.org/10.1517/17425247.2011.577063
Biddiscombe MF, Meah SN, Underwood SR, Usmani OS (2011) Comparing lung regions of interest in gamma scintigraphy for assessing inhaled therapeutic aerosol deposition. J Aerosol Med Pulm Drug Deliv 24(3):165–173. https://doi.org/10.1089/jamp.2010.0845
Boisson M, Jacobs M, Gregoire N, Gobin P, Marchand S, Couet W, Mimoz O (2014) Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother 58(12):7331–7339. https://doi.org/10.1128/AAC.03510-14
Athanassa ZE, Markantonis SL, Fousteri MZ, Myrianthefs PM, Boutzouka EG, Tsakris A, Baltopoulos GJ (2012) Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med 38(11):1779–1786. https://doi.org/10.1007/s00134-012-2628-7
Petitcollin A, Dequin PF, Darrouzain F, Vecellio L, Boulain T, Garot D, Paintaud G, Ternant D, Ehrmann S (2016) Pharmacokinetics of high-dose nebulized amikacin in ventilated critically ill patients. J Antimicrob Chemother 71(12):3482–3486. https://doi.org/10.1093/jac/dkw313
Herkner H, Muller MR, Kreischitz N, Mayer BX, Frossard M, Joukhadar C, Klein N, Lackner E, Muller M (2002) Closed-chest microdialysis to measure antibiotic penetration into human lung tissue. Am J Respir Crit Care Med 165(2):273–276. https://doi.org/10.1164/ajrccm.165.2.2106082
Stahle L, Arner P, Ungerstedt U (1991) Drug distribution studies with microdialysis. III: Extracellular concentration of caffeine in adipose tissue in man. Life Sci 49(24):1853–1858
Elmquist WF, Sawchuk RJ (1997) Application of microdialysis in pharmacokinetic studies. Pharm Res 14(3):267–288
Chaurasia CS (1999) In vivo microdialysis sampling: theory and applications. Biomed Chromatogr 13(5):317–332. https://doi.org/10.1002/(SICI)1099-0801(199908)13:5<317::AID-BMC891>3.0.CO;2-I
Lonnroth P, Jansson PA, Smith U (1987) A microdialysis method allowing characterization of intercellular water space in humans. Am J Phys 253(2 Pt 1):E228–E231
Nahar K, Gupta N, Gauvin R, Absar S, Patel B, Gupta V, Khademhosseini A, Ahsan F (2013) In vitro, in vivo and ex vivo models for studying particle deposition and drug absorption of inhaled pharmaceuticals. Eur J Pharm Sci 49(5):805–818. https://doi.org/10.1016/j.ejps.2013.06.004
Kampmeier T, Arnemann P, Hessler M, Rehberg S, Morelli A, Westphal M, Lange M, Van Aken H, Ertmer C (2017) Provision of physiological data and reference values in awake and anaesthetized female sheep aged 6–12 months. Vet Anaesth Analg. https://doi.org/10.1016/j.vaa.2016.03.005
Acknowledgements
The authors would like to acknowledge the contributions from the staff and administrators of the facilities scoped for the project as follows:
-
a)
Department of Pharmacology, University of Sydney, Australia.
-
b)
School of Veterinary Science, Gatton Complex, University of Queensland.
-
c)
The Medical Engineering and Research Facility (MERF), Queensland University of Technology.
Funding
Jayesh Dhanani would like to acknowledge the funding from The Prince Charles Hospital Foundation (MS2011-40) and The Royal Brisbane and Women’s Hospital Foundation grants (2012 and 2016). Jason Roberts wishes to recognise the funding from the Australian National Health and Medical Research Council for Centre of Research Excellence (APP1099452) and a Practitioner Fellowship (APP1117065). John F. Fraser acknowledges Queensland Health Research Fellowship and Australian National Health and Medical Research Council for Centre of Research Excellence.
Author information
Authors and Affiliations
Contributions
JD conceptualized, wrote, and edited the manuscript. JR helped in finalisingthe project, writing, and editing. JFF, JeC, JL, MC, KC and PT contributed to the writing and editing of the manuscript. JD and MC designed and refined the microdialysis model. BA and AK reviewed and refined the ovine model, edited the respective part of the paper. MB and SG refined the radiolabelled antibiotic aspect of the project and edited the paper. SD, JiC and KD refined the animal model and edited the paper. AB performed the statistical analyses for the individual studies. SW and SP reviewed, refined and edited the analytical aspect of the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The animal studies have ethics approval from the Queensland University of Technology animal ethics committee approval (Approval no. 1100000052) and University of Queensland animal ethics committee approval (Approval no. SOM/268/16/RBWH).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dhanani, J.A., Cohen, J., Parker, S.L. et al. A research pathway for the study of the delivery and disposition of nebulised antibiotics: an incremental approach from in vitro to large animal models. ICMx 6, 17 (2018). https://doi.org/10.1186/s40635-018-0180-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-018-0180-7