Abstract
Introduction
Gross hematuria caused by rupture of an artery in the urinary tract is a rare but potentially fatal condition. Iliac artery aneurysms, pelvic surgery with radiation, vascular reconstructive surgery, surgery for stenosis of the ureteropelvic junction, and transplantation are reported to be associated with this condition. In the vascular reconstructive surgery group, the most common etiology is rupture of the degenerated artery or synthetic graft in the ureter.
Case presentation
We present a case of rupture of the small anastomotic pseudoaneurysm at the proximal anastomosis of a right iliofemoral autogenous vein extra-anatomic graft in the urinary bladder. To our knowledge, this is the first report of a rupture of an autogenous vein graft in the urinary bladder. Our patient, a 24-year-old Albanian farmer, was admitted to the emergency department in severe hemorrhagic shock induced by exsanguinating hematuria. He underwent immediate surgery, during which direct sutures to the bladder were placed and the saphenous graft was replaced with a synthetic one. The patient recovered completely, was free of hematuria, and showed no signs of pathological communication between the urinary and arterial tracts on postoperative cystoscopy and computed tomographic angiography during 2 years of follow-up.
Conclusion
The incidence of artery-to-urinary tract fistulas is growing due to the increasing use of urologic and vascular surgery, pelvic oncologic surgery, and radiation therapy. In addition to fistulas involving a degenerated artery and ureter or synthetic grafts and ureter, they can also involve an autogenous vein graft and the urinary bladder. In our patient, the fistula was a result of erosion of the bladder from a pseudoaneurysm at the proximal anastomosis of an autogenous vein iliofemoral bypass in an extra-anatomic position. Open surgery remains the best treatment option, although there is increasing evidence of successful endovascular treatment.
Similar content being viewed by others
Introduction
Artery-to-urinary tract fistulas (AUFs) are rare, erosive defects that occur between the segments of the urinary tract and adjacent blood vessels or vascular grafts. From 1908, when first described by Moschcowitz [1], until 10 years ago, less than 150 cases were reported in the literature [2]. Primary fistulas, which account for less than 15% of reported AUFs, are usually associated with arteriovenous malformations and aneurysmal degeneration of the aorta and iliac arteries [3,4,5], whereas secondary fistulas, which account for all other reported AUFs, are associated with previous vascular surgery, pelvic oncologic surgery, and radiation therapy. All reported fistulas involved exclusively degenerated artery and ureter or synthetic graft and ureter [6,7,8]. To our knowledge, we report the first instance of the communication of an autogenous vein graft with the bladder.
Case presentation
Our patient was a 24-year-old Albanian man who was admitted to the Emergency Department of the University Hospital in Kosovo in severe hemorrhagic shock due to a massive hematuria. The patient is a farmer, does not smoke, and does not consume alcohol. He has no significant family and social history of medical relevance. Ten years ago, he had sustained a third-degree burn injury over approximately 70% of his body surface area and had been treated in a specialized center in a neighboring country. During that hospitalization, the patient had multiple venous lines placed in the groin and developed an infection that led to the rupture of the common femoral artery. To treat it, an autogenous vein extra-anatomic iliofemoral bypass was constructed. Since then, he had never been seen by a vascular surgeon.
Prior to his admission to our emergency department, he had two episodes of gross hematuria for which he was treated at the regional hospital. Diagnostic evaluation during earlier hospitalizations did not reveal the cause of bleeding. AUF was not considered on either of the occasions. The treatment was conservative and involved bladder lavage and blood transfusions. He was discharged on antibiotics, uroseptics, and iron supplements.
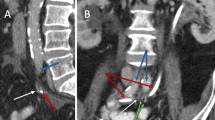
The possibility of communication between the arterial and urinary tracts was suspected on the basis of cystoscopy performed in the outpatient setting 2 days before the current admission (Fig. 1). The finding of the source of the bleeding at the right upper corner of the bladder, very close to the ureteral opening, raised the suspicion of possible AUF. The diagnosis was confirmed the next day, when contrast-enhanced magnetic resonance imaging showed proximity of a small pseudoaneurysm located at the proximal anastomosis of the enlarged extra-anatomic right iliofemoral autogenous vein graft and bladder (Fig. 2). The patient, who was free of bleeding, was referred to a vascular surgeon and admitted to the vascular surgery department. Several units of red blood cells and fresh frozen plasma (FFP) were ordered, along with antibiotics, and the patient was scheduled for elective surgery on the next day. Unfortunately, during the night, he experienced a third episode of exsanguinating bleeding and was transferred to the emergency department.
At the emergency department, he was confused and anxious, and his skin was pale, cold, and clammy. He was sweating and was breathing rapidly. His pulse on the peripheral arteries was weak, rapid, and thready. His fingernails and lips were blue, and his capillary refill time was 5 seconds. His blood pressure was 70/40 mmHg, heart rate 130 beats/minute, and peripheral capillary oxygen saturation 92%. His hematocrit was 19%, and his hemoglobin was 7 g/dl. His platelet count was normal, and his white blood cell count was slightly elevated (11.7 × 109/L). His glucose, cholesterol, urea, and creatinine concentrations were within normal range. His total bilirubin was moderately elevated (30.6 μmol/L), and his transaminase level was normal. He had a significantly high level of C-reactive protein (55.8 mg/L). His urine was full of blood cells. No serology or microbiology was performed. Hemodynamic resuscitation was initiated immediately. Two large-bore (16-gauge) intravenous catheters were inserted. Crystalloids and colloids were rapidly administered, and red blood cells and FFP were ordered.
Induction agents etomidate (0.3 mg/kg), fentanyl (3 μg/kg), and rocuronium (1.2 mg/kg) were administered. The patient was intubated and escorted to the operation room. Anesthesia was maintained with sevoflurane (0.7–1.3 minimum alveolar concentration), atracurium, and fentanyl. To achieve hemodynamic stability, vasopressors (dopamine 5–7 μg/minute) were used until several units of red blood cells and FFP were brought from the transfusion desk. To minimize the possibility of rebleeding, permissive hypotensive resuscitation was maintained.
The abdomen was opened employing a right extraperitoneal approach. After obtaining vascular control, the rupture site was reached through the native aneurysmal part of the venous graft and was closed with simple sutures (Fig. 3). Because of the severe scars on the skin and varicosity of the saphenous vein, we decided to perform a new bypass using a synthetic graft. The proximal anastomosis of the synthetic graft was placed on the iliac artery 5 cm above the site of the rupture, and the distal part of the graft was anastomosed in an end-to-side fashion with the existing autogenous vein graft, several centimeters before the site of the original distal anastomosis. The graft above the distal anastomosis was ligated (Fig. 4).
At the end of the operation, the patient was transferred to the intensive care unit. On the next day, he was extubated and transferred to the ward. The patient recovered completely, and postoperative cystoscopy showed no signs of pathological communication (Fig. 5). He was free of hematuria episodes for the whole postoperative period. Computed angiography performed 2 years after the surgery showed correct position of the graft with no complications (Fig. 6).
Discussion
Adding to previous reports of AUFs involving a degenerated artery or a synthetic graft with ureter [6,7,8], we report the first instance of a communication of an autogenous vein graft with bladder. We believe that the reason for this was a small pseudoaneurysm at the site of proximal anastomosis of the autologous vein in an extra-anatomic iliofemoral bypass that eroded the bladder.
There are several theories to explain the development of the pseudoaneurysm [9]. Infection [10,11,12,13,14,15,16], degeneration of the artery [10, 12, 17,18,19,20,21,22,23,24,25,26,27], aseptic necrosis at the anastomotic line [28, 29], extensive endarterectomy, [10, 12, 14, 30, 31], large anastomosis or patch [10, 14, 32], the way that the anastomosis was performed [14, 33, 34], the length of the graft [35], adhesion with surrounding structures [34], mismatch between vessels [36,37,38], and combinations of these situations have been widely discussed. It is not clear which of the aforementioned was responsible for the development of the pseudoaneurysm in our patient, although it is likely that the significantly larger autogenous vein graft may have caused mechanical stress on the proximal anastomosis and contributed to the formation of the pseudoaneurysm. Other contributing factors that should be considered are the extra-anatomic position of the graft and the age of the patient at the time of surgery (14 years old).
Regardless of origin, a typical presentation of AUFs includes different degrees of hematuria ranging from microscopic to exsanguinating hemorrhage. AUFs are found more frequently in women and tend to occur between 2 and 25 years after the initial surgical intervention. Mortality rates of 0–23% have been reported. However, without an accurate preoperative diagnosis, a worse prognosis has been reported (64% mortality rate) [39, 40].
The most common diagnostic tools are ultrasound, computed tomography, and magnetic resonance angiography, and cystoscopy [39, 40]. Surgical management of arterial–ureteral fistulas has several goals, namely hemorrhage control, restoration of vascular and urinary continuity, and resection of potentially infected tissue or prosthetic material [38, 40, 41]. Multiple treatment options regarding the arterial defect have been reported, with a recent trend toward an endovascular approach [42]. Open surgery alternatives are local reconstruction with arteriorrhaphy, patch closure, interposition graft, or bypass. There are reports of simple closure of the rupture site and excision of the graft without reconstruction that have been well tolerated [42,43,44,45,46,47]. Ureter repair is performed by reimplanting the ureter away from the vessels or by placing a nephrostomy tube. If the kidney is atrophic, nephrectomy and ureterectomy are performed [48].
The first description of endovascular treatment of AUFs was reported by Arap et al. [49] in 1965. Since then, multiple options of endovascular treatment of AUFs have been developed, including stent graft exclusion of the fistula and coil embolization of the affected artery with or without placement of a bypass graft for limb perfusion [42, 50,51,52,53].
Conclusion
The incidence of AUFs is growing due to an increase in urologic and vascular surgery, pelvic oncologic surgery, and radiation therapy. In addition to the fistulas involving degenerated artery and ureter or synthetic grafts and ureter, they can also involve autogenous vein graft and urinary bladder. In our patient, the fistula was a result of erosion of the bladder from a pseudoaneurysm at the proximal anastomosis of an autogenous vein iliofemoral bypass in an extra-anatomic position.
Open surgery aimed at hemorrhage control, restoration of vascular and urinary continuity, and resection of potentially infected tissue or prosthetic material remains the best therapeutic option for AUFs, even though there is increasing evidence of successful endovascular treatment.
Early recognition is crucial to success. This is why AUFs should be suspected in all patients with hematuria and a history of the aforementioned surgical and radiological procedures.
Availability of data and materials
The data are available under consideration of the corresponding author on reasonable request.
References
Moschcowitz AV. IX: simultaneous ligation of both external iliac arteries for secondary hemorrhage. Ann Surg. 1908;48:872–5.
van den Bergh RC, Moll FL, de Vries JP, Yeung KK, Lock TM. Arterio-ureteral fistula: 11 new cases of a wolf in sheep’s clothing. J Urol. 2008;179:578–81.
Seitz M, Waggershauser T, Khoder W. Congenital intrarenalarteriovenous malformation presenting with gross hematuria after endoscopic intervention: a case report. J Med Case Rep. 2008;2:326.
Carrafiello G, Laganà D, Peroni G, Mangini M, Fontana F, Mariani D, Piffaretti G, Fugazzola C. Gross hematuria caused by a congenital intrarenal arteriovenous malformation: a case report. J Med Case Rep. 2011;5:510.
Sountoulides P, Zachos I, Paschalidis K, Asouhidou I, Fotiadou A, Bantis A, Palasopoulou M, Podimatas T. Massive hematuria due to a congenital renal arteriovenous malformation mimicking a renal pelvis tumor: a case report. J Med Case Rep. 2008;2:144.
Van den Bergh RCN, Moll FL, De Vries JPPM, Lock TMTW. Arterioureteral fistulas: unusual suspects – a systematic review of 139 cases. Urology. 2009;74:251–5.
Mitterberger M, Frauscher F, Steppan I, Peschel R, Pinggera GM. Ureteroiliac fistula: a case report review of the literature. Cases J. 2009;2:62–6.
Oliveira N, Oliveria F, MotaPreto P, Cassio I. A primary arterial–ureteral fistula after anaorticbifemoral bypass. Int J Surg Case Rep. 2013;4(1):48–50.
Marković DM, Davidović LB, Kostić DM, Maksimović ZV, Cinara IS, Cvetković SD, Marković MD, Dragas MV. Anastomotic pseudoaneurysms [in Serbian]. Srp Arh Celok Lek. 2006;134(3–4):114–21.
Kim GE, Imporato AM, Nathan I, Riels TS. Dilatation of synthetic grafts and junctional aneurysms. Arch Surg. 1979;114:1296–303.
Sawyers JL, Jacobs JK, Sutton JP. Peripheral anastomotic aneurysms. Arch Surg. 1967;95:802–9.
Szilagyi DE, Smith RF, Elliot JP, et al. Anastomotic aneurysms after vascular reconstruction: problems of incidence, etiology and treatment. Surgery. 1975;78(6):800–16.
Watanabe T, Kusaba A, Kuma H, et al. Failure of Dacron arterial prostheses caused by structural defect. J Cardiovasc Surg. 1983;24:95–100.
Clark ET, Gewertz BL. Pseudoaneurysms. In: Rutherford RB, editor. Vascular surgery. 4th ed. Philadelphia: Saunders; 1994. p. 1153–61.
Gutman H, Zelinovski A, Reiss R. Ruptured anastomotic pseudoaneurysms after prosthetic vascular graft bypass procedures. J Med Sci. 1984;20(7):613–7.
Broyn T, Christensen O, Fossdal JE, Kordit KF, Kroese A, Myhre HO. Early complications with a new bovine arterial graft (Solcograft P). Acta Chir Scand. 1986;152:263–6.
Mulder EJ, van Bockel JH, Maas J, van den Akker PJ, Hermans J. Morbidity and mortality of reconstructive surgery of noninfected false aneurysms detected long after aortic prostetic reconstruction. Arch Surg. 1998;133(1):45–9.
DeBakey ME, Crawford ES, Morris GC, Cooley LA. Patch graft angioplasty in vascular surgery. J Cardiovasc Surg. 1963;3:106–41.
Tridico F, Zan S, PanierSuffat P, Contessa L, Bruno F, Caldart M. Femoral anastomotic pseudoaneurysms: the etiopathogenetic hypotheses and the therapy. Minerva Chir. 1992;47(1–2):37–40.
Morbidelli A, Caron R, Caldana G, Musazzi M, Capobianco M, Florianello F. Bilateral thrombosis of a femoral pseudoaneurysm. Minerva Chir. 1995;50(11):1013–8.
Levi N, Schroeder TV. Anastomotic femoral aneurysms: increase in interval between primary operation and aneurysm formation. Eur J Vasc Endovasc Surg. 1996;11:207–9.
Satiani B, Karmers M, Evans NE. Anastomotic arterial aneurysms. Ann Surg. 1980;192:674–82.
Nichols WK, Stanton M, Silver D, Keitzer WF. Anastomotic aneurysms following lower extremity revascularization. Surgery. 1980;88:366–74.
Sedwitz MM, Hye RJ, Stabile BE. The changing epidemiology of pseudoaneurysms: therapeutic implantations. Arch Surg. 1988;123:473–6.
Knox WG. Peripheral vascular and anastomotic aneurysms: a fifteen-year experience. Ann Surg. 1976;183:120–3.
Dorros G, Jaff MR, Parikh A, et al. In vivo crushing of an aortic stent enables endovascular repair of a large infrarenal aortic pseudoaneurysm. J Endovasc Surg. 1998;5(4):359–64.
Merrill EW, Salzam EW. Properties of material affecting the behavior of blood art their surfaces. In: Sawyer PN, Kaplitt MJ, editors. Vascular graft. New York: Appleton Century Crofts; 1978. p. 119–29.
Gaylis H. Pathogenesis of anastomotic aneurysms. Surgery. 1981;90(3):509–15.
Esolowski AS, Golaski WM, Saquwage LR, et al. Considerations in the development of small artery prostheses. Trans Amer Soc Artif Intern Organs. 1968;14:43–7.
Dubost C, Allary M, Olconomos N. Resection of an aneurysm of the abdominal aorta: reestablishment of the continuity by a preserved human arterial graft with result after five months. Arch Surg. 1952;64:405–8.
Nunn DB, Freeman MH, Hudgins L. Postoperative alternations in size of Dacron grafts. Ann Surg. 1979;189:741–4.
Edwards JM, Teefey SA, Zierler RE, Kohler TR. Intraabdominal paraanastomotic aneurysms after aortic bypass grafting. J Vasc Surg. 1992;15:344–53.
Stoney RJ, Albo EJ. False aneurysms occurring after arterial grafting operations. Am J Surg. 1965;110:153–61.
Dadgar L, Downs AR, Deng X, et al. Longitudinal forces acting at side-to-end and end-to-side anastomoses when a knitted polyester arterial prosthesis is implanted in the dog. J Investig Surg. 1995;8(3):163–78.
Read RC, Thompson BW. Uninfected anastomotic false aneurysms following arterial reconstruction with prosthetic grafts. J Cardiovasc Surg (Torino). 1975;16(5):558–61.
Sieswerda C, Skotnicki SH, Barentz JO, Heystraten FMJ. Anastomotic aneurysms – an underdiagnosed complication after aorto-iliac reconstructions. Eur J Vasc Surg. 1989;3:233–8.
McCann RL, Schwartz LB, Georgiade GS. Management of aortic graft complications. Ann Surg. 1993;217:729–34.
Bastounis E, Georgopoulos S, Maltezos C, Balas P. The validity of current vascular imaging methods in the evaluation of aortic anastomotic aneurysms developing after abdominal aortic aneurysm repair. Ann Vasc Surg. 1996;10:537–45.
Madoff DC, Gupta S, Toombs BD. Arterioureteral fistulas: a clinical, diagnostic, and therapeutic dilemma. AJR Am J Roentgenol. 2004;182:1241–50.
Krambeck AE, DiMarco DS, Gettman M, Segura JW. Ureteroiliac artery fistula: diagnosis and treatment algorithm. Urology. 2005;66:990–4.
Fox JA, Krambeck A, McPhail EF, Lightner D. Ureteroarterial fistula treatment with open surgery versus endovascular management: long-term outcomes. J Urol. 2011;185:945–50.
Malgor RD, Oderich GS, Andrews JC. Evolution from open surgical to endovascular treatment of ureteral–iliac artery fistula. J Vasc Surg. 2012;55:1072–80.
Bergqvist D, Parsson H, Sherif A. Arterio-ureteral fistula – a systematic review. Eur J Vasc Endovasc Surg. 2001;22:191–6.
Ferrante A, Manni R, Giustacchini M, Cotroneo A, Snider F. Arterioureteric fistula: successful treatment of two cases. Eur J Vasc Endovasc Surg. 2004;28:559–61.
Wu IH, Chueh SC, Liang PC. Simultaneous endovascular treatment of a ureteroiliac fistula and common iliac artery occlusion. Eur J Vasc Endovasc Surg. 2008;16:1–3.
Xiangyi Z, Yiwei L, Bin C, Xianyong Z, Xiaofeng Z, Yuehong S, Liping X. Severe hematuria after transurethral electrocoagulation in a patient with an arteriovesical fistula. BMC Urol. 2013;13:68.
Nobuhiro T, Yusuke N. Ruptured renal arteriovenous malformation successfully treated by catheter embolization: a case report. BMC Res Notes. 2014;7:19.
Takahashi Y, Hirai H, Sasaki Y, Shibata T, Bito Y, Suehiro S. Successful surgical treatment for rupture of an iliac artery aneurysm into a ureter. Ann Vasc Dis. 2009;2:58–61.
Arap S, Góes GM, de Freire JG, Nardy OW, Azevedo JR. Uretero-arterial fistula [in Portuguese]. Rev Paul Med. 1965;67:352–6.
Subiela JD, Balla A, Bollo J, Dilme JF, Soto Carricas B, Targarona EM, Rodriguez-Faba O, Breda A, Palou J. Endovascular management of ureteroarterial fistula: single institution experience and systematic literature review. Vasc Endovasc Surg. 2018;52(4):275–86.
Pillai AK, Anderson ME, Reddick MA, Sutphin PD, Kalva SP. Ureteroarterial fistula: diagnosis and management. Am J Roentgenol. 2015;204(5):W592–8.
Tselikas L, Pellerin O, Di Primio M, Arfi MB, Joskin J, Beyssen B, Thiounn N, Sapoval M. Uretero-iliac fistula: modern treatment via the endovascular route. Diagn Intervent Imaging. 2013;94(3):311–8.
Das A, Lewandoski P, Laganosky D, Walton J, Shenot P. Ureteroarterial fistula: a review of the literature. Vascular. 2016;24(2):203–7.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LJ, BA, and DK performed the surgery and analyzed and interpreted the data. VIJ, AK, AJ, and FV reviewed the literature. BG performed radiological studies. AGG provided perioperative care for the patient. All authors were major contributors to the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Committee of the University Clinical Center of Kosovo.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jaha, L., Ismaili-Jaha, V., Ademi, B. et al. Massive hematuria due to an autogenous saphenous vein graft and urinary bladder fistula in an extra-anatomic iliofemoral bypass: a case report. J Med Case Reports 13, 359 (2019). https://doi.org/10.1186/s13256-019-2300-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-019-2300-8









