Abstract
Background
Dyslipidaemia has always been regarded as the cornerstone of arteriosclerosis and is related to the pathogenesis of renal insufficiency. However, it is unclear which routinely available lipid parameter is related to urinary albumin to creatinine ratio (UACR). The purpose of this study was to examine the lipid abnormalities associated with UACR in the general population in China.
Methods
The present study was nested in an ongoing Risk Evaluation of cAncers in Chinese diabetic Individuals: A lONgitudinal (REACTION) study, which was designed to demonstrate the association of abnormal glucose metabolism with the risk of cancer in the Chinese population. This cross-sectional study included 34, 569 subjects (11, 390 males and 23, 179 females) from 8 different regional community cohorts, with an average age of 57.9 years. The UACR data were divided into the < 25% group, the 25–49% group, the 50–74% group, and the ≥ 75% group according to the quartile division of the centre where the subjects visited. The lipid classes were defined according to the guidelines for the prevention and treatment of dyslipidaemia in Chinese adults. Multiple logistic regression analysis was used to evaluate the association of the lipid parameters and UACR.
Results
Multivariable regression analysis revealed that compared with the other lipid parameters, triglycerides (TG) showed an adjusted odds ratio that was significant in model 1–4. This relationship was attenuated after adjusting for Haemoglobin A1c (HbA1c) and blood pressure (BP), but TG ≥ 2.3 mmol/L was still significantly associated with UACR in total subjects and in both men and women (OR: 1.131, 95% CI 1.065–1.203, P < 0.001 in total subjects; OR: 1.134, 95% CI 1.022–1.258, P = 0.017 in men; OR: 1.129, 95% CI 1.046–1.219, P = 0.002 in women). In the stratified analysis, elevated TG was significantly associated with increased urinary albumin in subjects with eGFR ≥ 90 mL/min per 1.73 m2, 5.6 ≤ FBG < 7.0 or 7.8 ≤ PBG < 11.1 mmol/L, 24 ≤ BMI < 28 kg/m2, 120 ≤ SBP < 140 and/or 80 ≤ DBP < 90 mmHg.
Conclusions
We conclude that high TG levels rather than total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, or non-high-density lipoprotein cholesterol levels are associated with UACR in the general population in China.
Similar content being viewed by others
Background
An elevated level of the urinary albumin to creatinine ratio (UACR) is not only a marker of renal dysfunction but also has been described as an independent predictor of cardiovascular disease in diabetic [1, 2] and non-diabetic patients [3, 4]. Moreover, recent findings have indicated that the UACR is more closely associated with diabetic retinopathy than the estimated glomerular filtration rate (eGFR) [5] and might be valuable in evaluating the risk for cognitive decline [6, 7]. Additionally, a robust body of literature has demonstrated that moderately increased albuminuria (UACR less than 30 mg/g) within the accepted normal range is associated with higher cardiovascular morbidity and mortality even in the general population [8, 9]. Moreover, every 3.01 mg/g (equivalent to 0.4 mg/mmol) increment in UACR conferred a 5.9% increase of major cardiovascular events [10].
The mechanisms by which increased UACR is linked to an increased risk of cardiovascular disease remain to be clarified, but one of the mechanisms is its link with atherogenic lipoproteins. Dyslipidaemia has always been regarded as the cornerstone of arteriosclerosis and the primary target of therapy according to international guidelines [11, 12]. In addition, it has been reported that dyslipidaemia associated with arteriosclerotic complications is the most common cause of death in chronic kidney disease (CKD) patients, and dyslipidaemia has also been shown to be an independent risk factor for the progression of CKD [13, 14]. Furthermore, a previous meta-analysis demonstrated that statins for treatment of dyslipidaemia may be beneficial for the reduction of albuminuria in CKD patients [15]. In practice, however, it is unclear which routinely available lipid measure is more applicable in estimation of kidney function. Research on this aspect has yielded controversial results, and most of these studies were in people with diabetes [16, 17]. Recently, a cross-sectional study was conducted in China to investigate the association between lipid parameters, albuminuria and chronic kidney disease. However, the study included only 9730 subjects, and the authors did not adjust for serum creatinine (Cr) and liver function in the logistic regression, which were thought to potentially skew results [18].
Lipids remain part of the conventional risk factors for UACR and atherosclerosis, especially in the general population. Therefore, the aim of this study was to comprehensively assess the association of all routine lipid parameters and albuminuria in the general population in 8 different regions of China.
Methods
Study population and design
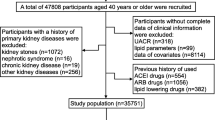
The present study was nested in A lONgitudinal REACTION (Risk Evaluation of cAncers in Chinese diabeTic Individuals) study, which was designed to investigate the association of type 2 diabetes mellitus (T2DM) and pre-diabetes with the risk of cancer in the Chinese population, described previously [19]. The REACTION study was set up as a multicentre prospective observational study, and our study population was from the eight of the centres. A total of 53, 639 participants aged 40 years or older were recruited and invited to participate by questionnaire survey between March and December 2012. (Dalian 10140, Lanzhou 10026, Guangzhou 9743, Luzhou 8105, Shanghai 6821, Guangxi 5831, Zhengzhou 1978, Wuhan 995). Subjects who had been diagnosed with primary kidney diseases, used ACEI/ARB medicines, used lipid-lowering drugs or whose data missing and/or included outliers were excluded. A total of 36, 352 subjects had complete data. After the propensity score matching, the remaining 34, 569 eligible subjects (11, 390 males and 23, 179 females) were enrolled in this cross-sectional study (Fig. 1).
All investigators received extensive training related to the study questionnaire and outcome measures before carrying out the investigation. The study protocol was approved by the Committee on Human Research at Rui-Jin Hospital affiliated with the School of Medicine, Shanghai Jiao Tong University. Written informed consents were obtained from all participants before data collection.
Clinical evaluation and laboratory measurements
All participants received comprehensive examinations that included a detailed questionnaire, anthropometric measurement, blood collection, and a standard 75-g oral glucose tolerance test (OGTT) or steamed-bread meal test. The self-administered questionnaire covered the history of diabetes, hypertension, hyperlipidaemia, acute/chronic nephritis, nephritic syndrome, kidney stones, cardiovascular diseases [CVD, including myocardial infarction (MI), stoke and coronary heart disease (CHD)], diabetes, alcohol intake, and smoking habits. Alcohol intake was classified as either consumption nearly/more than once a week currently or not; smoking habit was classified as smoking more than once a day or not.
Body mass indices (BMI) were calculated as the weight in kilograms divided by the height in metres squared (kg/m2). Repeated blood pressure (BP) was measured three times consecutively by the same observer in 5-min intervals. The average of three blood pressure measurements was used for analysis. Blood samples were collected by venipuncture, and all participants were told to fast for at least 10 h before the test. Participants with or without a history of diabetes underwent a 100 g steamed-bread meal test or 75 g oral glucose tolerance test, respectively. Fasting blood glucose (FBG), 2 h post-load blood glucose (PBG), serum triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), Haemoglobin A1c (HbA1c), alanine transferase (ALT), aspartate transferase (AST), gamma-glutamyl transferase (GGT), and Cr were measured in each centre. Non-high-density lipoprotein cholesterol (non-HDL-C) levels were calculated from the difference between serum TC and HDL-C.
The estimated glomerular filtration rate (eGFR) was expressed in mL/min per 1.73 m2 by the formula eGFR = 186 × [serum creatinine × 0.011] − 1.154 × [age] − 0.203 × [0.742 if female] × 1.233, where serum creatinine was expressed as μmol/L and 1.233 was the adjusting coefficient for the Chinese population. This formula is according to the abbreviated Modification of Diet in Renal Disease (MDRD), which was recalibrated for Chinese population [20].
Definition of UACR group and blood lipids categories
The spot first morning urine samples were collected to measure the concentration of urine albumin and creatinine. UACR was calculated by dividing the urinary albumin concentrations by the urinary creatinine concentrations. The 8 centres adopted different UACR measurement methods, therefore, the normal value range and unit of the measurement were not unified. To avoid this problem, we divided UACR as classification variables for analysis. The UACR data were divided into the < 25% group, the 25–49% group, the 50–74% group, and the ≥ 75% group according to the quartile division of the centre where the subject visited. (UACR percentiles).
According to the guidelines for the prevention and treatment of dyslipidaemia in Chinese adults (revised in 2016), we classified blood lipids into the following categories. TC was grouped into three categories (appropriate: < 5.2 mmol/L, borderline high: 5.2–6.1 mmol/L, and high: ≥ 6.2 mmol/L), TG was grouped into three categories (appropriate: < 1.7 mmol/L, borderline high: 1.7–2.2 mmol/L, and high: ≥ 2.3 mmol/L), LDL-C was grouped into three categories (appropriate: < 3.4 mmol/L, borderline high: 3.4–4.0 mmol/L, and high: ≥ 4.1 mmol/L), non-HDL-C were grouped into three categories (appropriate: < 4.1 mmol/L, borderline high: 4.1–4.8 mmol/L, and high: ≥ 4.9 mmol/L) and HDL-C was grouped into two categories (normal: ≥ 1.0 mmol/L and low: < 1.0 mmol/L).
Statistical analysis
The statistical analysis was performed using SPSS version 24.0 (IBM, Chicago, IL, USA). Continuous variables were presented as the means ± the standard deviations (SD) with the exception of skewed variables, which were presented as medians (interquartile ranges). Categorical variables were expressed as numbers (proportions). Differences in the continuous variables among the four subgroups of UACR were tested by one-way analysis of variance (ANOVA) followed by multiple comparison test using least significant difference (LSD). The χ2 test was used when the variables were categorical. Cardiovascular diseases (yes/no), smoking status and drinking status (non-current/current) were fitted as categorical variables.
The measured lipid parameters (i.e., TC, TG, HDL-C and LDL-C) and the calculated non-HDL-C were divided into groups according to Guidelines and the associations of these parameters among the quartiles of UACR were tested by ordered logistic regression analysis, performed in separate genders. Multivariate adjusted logistic regression analysis was also carried out to control potential confounders for determining the associations of lipid parameters with UACR in five models. Model 1 was adjusted for age and centres. Model 2 was further adjusted for ALT, AST, GGT and eGFR. Model 3 was further adjusted for current smoking, drinking status, and previously diagnosed CVD. Model 4 was further adjusted for BMI. Model 5 was further adjusted for HbA1c, SBP and DBP.
The relationship between TG and UACR was also explored within subgroups that were stratified by the level of eGFR (G1: eGFR ≥ 90 mL/min per 1.73 m2, G2: 60 ≤ eGFE < 90 mL/min per 1.73 m2, G3: eGFR < 60 mL/min per 1.73 m2), the level of BMI (Underweight: BMI < 18.5 kg/m2, Normal weight 18.5 ≤ BMI < 24 kg/m2, Overweight 24 ≤ BMI < 28 kg/m2, Obese BMI ≥ 28 kg/m2), the level of blood glucose (Normal: FBG < 5.6 and PBG < 7.8 mmol/L, Pre-diabetes: 5.6 ≤ FBG < 7.0 or 7.8 ≤ PBG < 11.1 mmol/L, Diabetes: FBG ≥ 7.0 or PBG ≥ 11.1 mmol/L), and level of blood pressure (Normal blood pressure: systolic blood pressure (SBP) < 120 and diastolic blood pressure (DBP) < 80 mmHg, High-normal blood pressure: 120 ≤ SBP < 140 and/or 80 ≤ DBP < 90mmHg, Hypertension: SBP ≥ 140 or DBP ≥ 90mmHg). In the stratified analyses, we separately examined feasible associated factors that could modify the relationship between albuminuria and lipid measures. Interaction analyses between TG and possible confounding factors were also conducted among the UACR groups. The odds ratios (OR) and the corresponding 95% confidence intervals (95% CI) were calculated. All statistical tests were two-sided, and P values < 0.05 were considered statistically significant.
Results
Clinical characteristics of the study population
A total of 34,569 subjects (11,390 men and 23,179 women) with a mean age (SD) of 57.9 (9.39) years were recruited. Table 1 shows the clinical and biochemical characteristics according to quartiles of UACR. There were 1127 (3.3%) participants with CHD, 387 (1.1%) participants with stroke, and 115 (0.3%) participants with MI. It can be seen that the incidence of cardiovascular disease increases with the increased UACR. The age of the participants increased as the UACR increased and the high quartile of UACR was characterized by significantly higher levels of blood glucose (0′,120′), BP, HbA1c, BMI, TC, LDL-C, non-HDL-C and TG. Furthermore, the eGFR decreased as the UACR increased.
Associations of lipid parameters with quartiles of UACR
Tables 2, 3 and 4 shows OR and 95% CI of the UACR quartiles with the categories of TG, TC, LDL-C, HDL-C, and non-HDL-C after adjusting for multiple confounders, separated into men and women. As seen in these tables, compared with other lipid parameters, only TG showed a significant adjusted odds ratio for all subjects and both sexes in model 1–4. This relationship was attenuated after adjusting for HbA1c and BP, but TG ≥ 2.3 mmol/L was still significantly associated with UACR in both men and women whereas TC, LDL-C, HDL-C, and non-HDL-C were not (OR: 1.131, 95% CI 1.065–1.203, P < 0.001 in total subjects; OR: 1.134, 95% CI 1.022–1.258, P = 0.017 in men; OR: 1.129, 95% CI 1.046–1.219, P = 0.002 in women). Although TC ≥ 6.2 mmol/L was significantly associated with UACR in the fully-adjusted model, the association was only seen in women, and it was not as remarkable as the association with TG (Table 3). No significant and independent association between non-HDL-C or LDL-C and UACR.
Associations between lipid parameters and UACR in people with LDL-C < 2.6 mmol/L or HDL-C > 1.0 mmol/L
According to the guidelines for the prevention and treatment of dyslipidaemia in Chinese adults (revised in 2016), we divided the population into two groups, LDL-C < 2.6 mmol/L (ideal value) and HDL-C > 1.0 mmol/L (low risk value). As shown in Table 5, high TG levels were still significantly associated with proteinuria excretion even if LDL-C was well controlled below 1.8 mmo/L or HDL-C was at a low risk level. No significant association was found in other lipid parameters.
Associations of TG with quartiles of UACR and stratified analyses for different levels of eGFR, BMI, blood glucose and blood pressure
To verify the stability of such results, we conducted stratified analyses in the various subgroups as shown in Table 6. These results indicate that compared with subjects with TG levels < 1.7 mmol/L, subjects with TG levels ≥ 2.3 mmol/L have the most significant association with UACR, especially in the pre-diabetes population (5.6 ≤ FBG < 7.0 or 7.8 ≤ PBG < 11.1 mmol/L). However, no significant relationship was found in either the normal population (FBG < 5.6 and PBG < 7.8 mmol/L) or the diabetic population (FBG ≥ 7.0 or PBG ≥ 11.1 mmol/L). Similar results were seen in people with borderline high blood pressure (120 ≤ SBP < 140 and/or 80 ≤ DBP < 90 mmHg) and those who were overweight (24 ≤ BMI < 28 kg/m2). We found a significant interaction between TG and blood glucose. Therefore, we further divided the pre-diabetic population into the impaired fasting glucose group (IFG: 5.6 ≤ FBG < 7.0 mmol/L and PBG < 7.8 mmol/L) and the impaired glucose tolerance group (IGT: FBG < 7.0 mmol/L and 7.8 ≤ PBG < 11.1 mmol/L) to observe the effect of fasting and postprandial blood glucose on this interaction. As shown in Additional file 1: Table S2, there was a significant relationship between TG and UACR in IFG group.
To better discuss the association of TG with UACR in different renal functions, we divided eGFR into three groups in Table 6. There was no significant association between TG and UACR in G2 stage (60 ≤ eGFR < 90 mL/min per 1.73 m2). However, when eGFR was more than 90 mL/min per 1.73 m2, the increase of triglyceride was significantly associated with UACR, while when eGFR was less than 60 mL/min per 1.73 m2, this relationship was at the borderline significant level. We also analyzed the relationship between all lipid indices and eGFR, as shown in Additional file 1: Table S1.
Discussion
Main findings
The main findings of this study suggested that among the lipid parameters, only TG was significantly associated with UACR in both men and women, whereas TC, LDL-C, HDL-C, and non-HDL-C were not. This association was consistently shown in the multiple regression models after adjusting for a wide spectrum of biochemical and lifestyle risk factors. To the best of our knowledge, the present study is the first multicentre, large sample clinical survey about the relationship between lipid parameters and UACR in a Chinese general population. After controlling for HbA1c and BP levels, the correlation between TG and UACR was weakened, indicating that HbA1c and BP levels added to the risk of proteinuria in this study. Further stratification showed that people with borderline values of BMI, blood glucose and BP had higher risks of urinary albumin when TG ≥ 2.3 mmol/L. Therefore, such people should be vigilant about the detection, avoidance and treatment of traditional risk factors.
Factors associated with TG and UACR
This study also analyzed the interaction between TG and possible confounding factors in the UACR group. We found that there was a significant interaction between TG and blood glucose, especially in the group with impaired fasting glucose (P = 0.016, Additional file 1: Table S2). This finding suggests that FBG is more closely associated with TG and UACR in pre-diabetic populations. The results are consistent with another study of a Mexican population demonstrated that higher triglyceride levels, greater waist circumference, and smoking are risk factors associated to diabetic kidney disease [21]. In addition, a Japanese study also demonstrated that not only fasting TG and FBG, but also the management of postprandial TG may have important significance in preventing the progression of type 2 diabetic nephropathy [22].
Decreased β-cell function and insulin resistance (IR) are considered central events in the development of T2DM. TG overload in islets impairs the function of β cells and interferes with glucose metabolism [23, 24]. Recently, a prospective study reported that changes in TG have a unidirectional relationship with peripheral IR, which provides evidence for the early prevention of IR by improving dyslipidaemia [25]. Furthermore, study of patients with abnormal glucose metabolism added that after appropriate treatment of high TG and high FBG level by n-3 fatty acids in patients with impaired glucose metabolism, the ability of insulin secretion was improved [26]. An individual can have pre-diabetes without knowledge or diagnosis of it for many years. Unfortunately, they may start to develop complications like diabetic nephropathy during this time even when asymptomatic. In reality, this is often the case in most chronic diseases until the symptoms worsen to a point that it affects the individual [27]. The present findings may provide evidence for early prevention of diabetic nephropathy by improving hypertriglyceridemia [28].
Interestingly, we noted that higher eGFR levels had a closer link with TG and UACR in our study. Glomerular hyperfiltration (GH) has been reported as a predictor of overt diabetic nephropathy [29]. An estimated 70% and 50% of patients with type 1 and 2 diabetes, respectively, develop GH early in their disease [30]. This may support the observations from Serena Low et al. [31] on the relationship between baseline hyperfiltration and rapid renal progression in T2DM among multi-ethnic Asians population. It is believed that GH is caused first by alteration in tubule glomerular feedback and the activation of vasoactive mediators which increase glomerular capillary pressure and lead to secondary increases in GFR (detected as GH) [32]. The exact mechanisms have not been elucidated completely but there is evidence suggesting that amelioration of GH by blocking the renin-angiotensin aldosterone system may confer renoprotection. Therefore, it would be important for the clinic to identify individuals with GH and to intervene at the right time.
Additionally, previous studies have shown that eGFR has a U-shaped relationship with all-cause mortality, indicating the importance of both high and low eGFR [9, 33]. However, the association between TG and UACR in low eGFR group was at the borderline significant level (P = 0.065) in present study. This difference may be explained, in part, by insufficient sample size of low eGFR group in our study. Further large sample or prospective studies are necessary to clarify the association of TG with UACR in different levels of eGFR.
Lipid parameters, atherosclerosis and proteinuria
It was widely accepted that high serum levels of LDL-C play a crucial role in the initiation and progression of atherosclerosis. Moreover, in the ACC/AHA and ESC/EAS guidelines, LDL-C is recommended as the most important lipid risk factor and therapeutic target for cardiovascular disease [34, 35]. However, LDL-C was not found to be a good indicator for albuminuria in present research (Tables 2, 3 and 4). It should be noted that measurement of LDL-C concentration has neglected the impact of other highly atherogenic particles such as very low-density lipoprotein (VLDL), and intermediate-density lipoprotein (IDL). Lipoprotein (a) and lipoprotein (b), which are called remnant cholesterol, also contribute to the development of atherosclerosis [36]. Furthermore, Assmann et al. have proved that despite currently available optimal LDL-C lowering therapies, a worrisome number of clinical events still occur [37, 38]. In fact, even if LDL was well controlled below 1.8 mmo/L, hypertriglyceridemia was still significantly associated with proteinuria excretion in this study (OR: 1.144, 95% CI 1.030–1.269, P = 0.012, Table 5). And the contribution of other lipid subfractions is increasingly being recognized [39, 40].
Interestingly, we found that HDL-C < 1.0 mmol/L was significantly associated with increased UACR in men after adjustment for many confounders, but this association was abrogated after additional adjustment for HbA1c and BP. High levels of HDL-C were thought to be protective against the development of atherosclerosis, and a low HDL-C level was associated with increased risk of CHD [41]. Additionally, some researchers hold that elevated triglyceride concentrations are strongly associated with low concentrations of HDL-C [42]. However, similar results were not observed in the present study. In fact, we used logistic regression to adjust for HDL-C (both separately and simultaneously), LDL-C, non-HDL-C, TC, BMI, BP, HbA1c, eGFR and other traditional risk factors for arteriosclerosis to reduce the possibility that these factors would confound our results. However, TG remained independently significantly associated with albuminuria. Apart from this, large outcome trials using fibrate or cholesterol ester transfer protein (CETP) inhibitors were designed to increase HDL-C and reduce the incidence of cardiovascular events. However, the overall results were negative or only positive in the subgroup. Thus, low HDL-C was not causally linked to atherosclerotic events on a population level. In fact, low HDL-C may simply be a very good indicator for an increased concentration of triglyceride-rich lipoproteins (TGRL) [43].
The understanding from recent genetic studies and randomized trials that low HDL-C might not be a cause of atherosclerotic disease as originally thought has generated renewed interest in elevated triglycerides [44]. Meta-analyses have supported the findings that high concentrations of TG were associated with increased risk of atherosclerotic disease even after adjustment for HDL-C concentrations [45]. Two reports from Korea also pointed out that TG has a strong association with arteriosclerosis in Korean adults [46, 47]. In addition, genetic studies strongly support the theory that high concentrations of TGRL or remnant cholesterol are causal risk factors for cardiovascular disease [48, 49]. It has been reported that a doubling of genetically elevated non-fasting triglyceride concentrations due to APOA5 genetic variants was associated with a 1.9-fold increased risk of myocardial infarction [50]. Specifically, the lipoprotein lipase (LPL) pathway and its reciprocal regulators apoA-V and apoC-III have been found to have remarkable associations with both TG and CHD [51].
The association and potential physiopathological mechanisms between TG and UACR
The role of dyslipidaemia in the development of albuminuria is still controversial, and the results of related studies have been inconsistent so far. One study of 275 Taiwanese cases reported that ApoB was the highest risk factor for albuminuria in both the diabetic men and the diabetic women [52]. However, the cases in this study involved early stage albuminuria, and the Cr levels were lower than 1.2 mg/dL. The exclusion of patients with elevated levels of Cr made the research results less reliable. Another prospective study from the Steno Diabetes Centre reported the baseline TC but not HDL was considered to be an independent risk factor for both micro-albuminuria and macro-albuminuria in type 2 diabetic patients. However, this study evaluated patients with more advanced renal disease [53].
Our findings are consistent with a Taiwanese study that recruited more participants (1026 males, 1323 females) and concluded that TG increased significantly throughout the 3 stages of albuminuria in Taiwanese Type 2 diabetic patients [54]. Additionally, an American study citing data from CACTI Study also showed that in adults with type 1 diabetes, fasting TG independently predicted higher odds of both coronary artery calcification (CACp) and incident albuminuria over 6 years, whereas LDL-C, HDL-C, non-HDL-C and TC did not. However, most of these studies were in people with diabetes. In our study, we confirmed the association between TG and UACR in the general population in 8 regions of China. Compared with the above studies, our study population was much larger, which allowed a careful control for the potential confounding effects using a stratified analysis and detected significant associations between TG and UACR in different levels of eGFR, BMI, blood glucose and BP.
A lipid profile includes measurement of the total amount of the two most important lipids in the plasma compartment–cholesterol and triglycerides. Lipoproteins include the smallest lipoproteins, HDL; medium-sized lipoproteins, LDL; and the largest lipoproteins, triglyceride-rich lipoproteins (remnants). For clinical reasons, the cholesterol content in these lipoprotein classes was reported as: HDL cholesterol, LDL cholesterol, and remnant cholesterol. We defined remnant cholesterol as the cholesterol content of all triglyceride-rich lipoproteins, i.e., chylomicron remnants, VLDL, and IDL.
Although the underlying pathophysiological mechanisms responsible for lipid-induced renal damage have yet to be uncovered, several studies suggest novel mechanisms by which TG may affect glomerular and tubular cell function. Plasma TG levels are known to correspond with the levels of TGRLs and their remnants [55]. The infiltration of TGRL into the glomerular endothelium and mesangial cells can trigger a cascade of events, including TGF-β pathway activation, monocyte chemoattractant production, adhesion molecule expression, and release of reactive oxygen species, that lead to early glomerular injury [56]. Once in the intima, LPL activity at the surface of remnants, either at the vascular endothelium or within the intima, leads to liberation of free fatty acids, monoacylglycerols, and other molecules, as well as foam cell formation, each of which could cause local injury and inflammation [57]. There is evidence that cellular damage by fatty acid accumulation in the kidney is particularly severe in podocytes, leading to apoptosis and resulting in glomerulosclerosis [58].
Limitations
Our study benefited from a large aggregation of multiple community-based samples, and the distribution of different regions in China was generally representative. However, some limitations existed. First, due to the different UACR measurement methods that were adopted by the 8 centres, the measurement units of UACR were not unified. As a result, the UACR could not be expressed as a continuous variable in the statistical analyses. However, the relationship between TG and UACR persisted after controlling for traditional risk factors in ordinal regression analysis. Second, although we excluded subjects who used ACEI/ARB and lipid-lowering drugs, we did not investigate other medications which might affect this relationship. Therefore, we could not eliminate the possible effect of medications on the present findings. Third, we evaluated the urinary albumin excretion on a spot morning urine sample. We admitted that multiple samples would provide more stable results for albumin excretion. However, it was reported that the results of spot urine samples correlate well with those of 24 h or multiple urine samples. The use of spot samples for assessing urinary ACR is more convenient and therefore recommended as a reliable alternative to perform in large epidemiological specimen collection [59]. Finally, as a cross-sectional study, we can only establish associations not cause. Further prospective follow-up studies are needed to fully ascertain the mechanisms underlying the association between dyslipidaemia and albuminuria.
Conclusion
In summary, we observed that high TG levels rather than total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, or non-high-density lipoprotein cholesterol levels are associated with UACR in the general population in China. People whose eGFR ≥ 90 mL/min per 1.73 m2 and BMI, blood glucose, or BP were borderline abnormal were more likely to have high risk of urinary albumin when TG ≥ 2.3 mmol/L. Given the clinical correlations with dyslipidaemia and proteinuria, it is important to take effective methods to improve the dyslipidaemia to decrease the risk of cardiovascular mortality and CKD progression. We believe that targeting lipid metabolism disorders in renal disease may increase the chance of successful drug discovery in the field of proteinuric kidney diseases.
Abbreviations
- UACR:
-
urinary albumin to creatinine ratio
- TG:
-
triglycerides
- HbA1c:
-
haemoglobin A1C
- BP:
-
blood pressure
- TC:
-
total cholesterol
- LDL-C:
-
low-density lipoprotein cholesterol
- HDL-C:
-
high-density lipoprotein cholesterol
- non-HDL-C:
-
non-high-density lipoprotein cholesterol
- eGFR:
-
estimated glomerular filtration rate
- CKD:
-
chronic kidney disease
- T2DM:
-
type 2 diabetes mellitus
- ACEI:
-
angiotensin converting enzyme inhibitor
- ARB:
-
angiotensin II receptor blocker
- CVD:
-
cardiovascular diseases
- MI:
-
myocardial infarction
- CHD:
-
coronary heart disease
- Cr:
-
serum creatinine
- FBG:
-
fasting blood glucose
- PBG:
-
2 h post-load blood glucose
- ALT:
-
alanine transferase
- AST:
-
aspartate transferase
- GGT:
-
gamma-glutamyl transferase
- SBP:
-
systolic blood pressure
- DBP:
-
diastolic blood pressure
- ACC/AHA:
-
American College of Cardiology and American Heart Association
- ESC/EAS:
-
European Society of Cardiology and European Atherosclerosis Society
- IR:
-
insulin resistance
- IFG:
-
impaired fasting glucose
- IGT:
-
impaired glucose tolerance
- GH:
-
glomerular hyperfiltration
- TGRL:
-
triglyceride-rich lipoproteins
References
Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310(6):356–60.
Scirica BM, Mosenzon O, Bhatt DL, Udell JA, Steg PG, McGuire DK, Im K, Kanevsky E, Stahre C, Sjostrand M, et al. Cardiovascular outcomes according to urinary albumin and kidney disease in patients with type 2 diabetes at high cardiovascular risk: observations from the SAVOR-TIMI 53 trial. JAMA Cardiol. 2018;3(2):155–63.
Wachtell K, Ibsen H, Olsen MH, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlof B, Devereux RB, Beevers G, de Faire U, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139(11):901–6.
Romundstad S, Holmen J, Kvenild K, Hallan H, Ellekjær H. Microalbuminuria and all-cause mortality in 2089 apparently healthy individuals: a 4.4-year follow-up study. The Nord-Trøndelag Health Study (HUNT), Norway. Am J Kidney Dis. 2003;42(3):466–73.
Romero-Aroca P, Baget-Bernaldiz M, Navarro-Gil R, Moreno-Ribas A, Valls-Mateu A, Sagarra-Alamo R, Barrot-De La Puente JF, Mundet-Tuduri X. Glomerular filtration rate and/or ratio of urine albumin to creatinine as markers for diabetic retinopathy: a ten-year follow-up study. J Diabetes Res. 2018;2018:5637130.
Ekblad LL, Toppala S, Johansson JK, Koskinen S, Sundvall J, Rinne JO, Puukka P, Viitanen M, Jula A. Albuminuria and microalbuminuria as predictors of cognitive performance in a general population: an 11-year follow-up study. J Alzheimers Dis. 2018;62(2):635–48.
Takahashi S, Tanaka F, Yonekura Y, Tanno K, Ohsawa M, Sakata K, Koshiyama M, Okayama A, Nakamura M. The urine albumin-creatinine ratio is a predictor for incident long-term care in a general population. PLoS ONE. 2018;13(3):e0195013.
Arnlov J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D’Agostino RB, Vasan RS. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112(7):969–75.
Chronic Kidney Disease Prognosis C, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81.
Valmadrid CT, Klein R, Moss SE, Klein BE. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med. 2000;160(8):1093–100.
Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285(19):2486–97.
Rana JS, Liu JY, Moffet HH, Solomon MD, Go AS, Jaffe MG, Karter AJ. Metabolic dyslipidemia and risk of coronary heart disease in 28,318 adults with diabetes mellitus and low-density lipoprotein cholesterol < 100 mg/dl. Am J Cardiol. 2015;116(11):1700–4.
Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58(1):293–301.
Sun K, Lin D, Li F, Qi Y, Feng W, Yan L, Chen C, Ren M, Liu D. Fatty liver index, albuminuria and the association with chronic kidney disease: a population-based study in China. BMJ Open. 2018;8(1):e019097.
Douglas K, O’Malley PG, Jackson JL. Meta-analysis: the effect of statins on albuminuria. Ann Intern Med. 2006;145(2):117–24.
Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, Group US. Risk factors for renal dysfunction in type 2 diabetes: U.K. prospective diabetes study 74. Diabetes. 2006;55(6):1832–9.
Tien KJ, Tu ST, Chen HC, Hsiao JY, Hsieh MC. Triglycerides are independently associated with albuminuria in Taiwanese Type 2 diabetic patients. J Endocrinol Invest. 2012;35(9):800–3.
Sun K, Lin D, Li F, Huang C, Qi Y, Xue S, Tang J, Yang C, Li Y, Ren M, et al. Discordant associations of lipid parameters with albuminuria and chronic kidney disease: a population-based study. Lipids Health Dis. 2015;14:152.
Ning G, Reaction Study G. Risk evaluation of cancers in Chinese diabeTic individuals: a lONgitudinal (REACTION) study. J Diabetes. 2012;4(2):172–3.
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–44.
Velazquez-Lopez L, Hernandez-Sanchez R, Roy-Garcia I, Munoz-Torres AV, Medina-Bravo P, Escobedo-de la Pena J. Cardiometabolic risk indicators for kidney disease in Mexican patients with type 2 diabetes. Arch Med Res. 2018;49(3):191–7.
Kitaoka K, Takenouchi A, Tsuboi A, Fukuo K, Kazumi T. Association of postbreakfast triglyceride and visit-to-visit annual variation of fasting plasma glucose with progression of diabetic nephropathy in patients with type 2 diabetes. J Diabetes Res. 2016;2016:4351376.
Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44(8):863–70.
Zhang M, Wang B, Liu Y, Sun X, Luo X, Wang C, Li L, Zhang L, Ren Y, Zhao Y, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: the Rural Chinese Cohort Study. Cardiovasc Diabetol. 2017;16(1):30.
Han T, Cheng Y, Tian S, Wang L, Liang X, Duan W, Na L, Sun C. Changes in triglycerides and high-density lipoprotein cholesterol may precede peripheral insulin resistance, with 2-h insulin partially mediating this unidirectional relationship: a prospective cohort study. Cardiovasc Diabetol. 2016;15(1):154.
Sawada T, Tsubata H, Hashimoto N, Takabe M, Miyata T, Aoki K, Yamashita S, Oishi S, Osue T, Yokoi K, et al. Effects of 6-month eicosapentaenoic acid treatment on postprandial hyperglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly-diagnosed impaired glucose metabolism patients with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc Diabetol. 2016;15(1):121.
De Simone G, Wang W, Best LG, Yeh F, Izzo R, Mancusi C, Roman MJ, Lee ET, Howard BV, Devereux RB. Target organ damage and incident type 2 diabetes mellitus: the Strong Heart Study. Cardiovasc Diabetol. 2017;16(1):64.
Liu YZ, Wang K, Li X, Chen B. Type II diabetes patients with proteinuria and the recovery of their metabolic parameters. Minerva Endocrinol. 2016;41(4):486–9.
Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52(4):691–7.
Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. 2012;8(5):293–300.
Low S, Tai ES, Yeoh LY, Liu YL, Liu JJ, Tan KH, Fun S, Su C, Zhang X, Subramaniam T, et al. Onset and progression of kidney disease in type 2 diabetes among multi-ethnic Asian population. J Diabetes Complications. 2016;30(7):1248–54.
Sasson AN, Cherney DZ. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes. 2012;3(1):1–6.
Tonelli M, Klarenbach SW, Lloyd AM, James MT, Bello AK, Manns BJ, Hemmelgarn BR. Higher estimated glomerular filtration rates may be associated with increased risk of adverse outcomes, especially with concomitant proteinuria. Kidney Int. 2011;80(12):1306–14.
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–934.
Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058.
Fujioka Y, Ishikawa Y. Remnant lipoproteins as strong key particles to atherogenesis. J Atheroscler Thromb. 2009;16(3):145–54.
Deedwania P, Barter P, Carmena R, Fruchart J-C, Grundy SM, Haffner S, Kastelein JJP, LaRosa JC, Schachner H, Shepherd J, et al. Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. The Lancet. 2006;368(9539):919–28.
Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–90.
Tani S, Yagi T, Atsumi W, Kawauchi K, Matsuo R, Hirayama A. Relation between low-density lipoprotein cholesterol/apolipoprotein B ratio and triglyceride-rich lipoproteins in patients with coronary artery disease and type 2 diabetes mellitus: a cross-sectional study. Cardiovasc Diabetol. 2017;16(1):123.
Zheng T, Ge B, Liu H, Chen B, Qin L, Xiao L, Song J. Triglyceride-mediated influence of serum angiopoietin-like protein 8 on subclinical atherosclerosis in type 2 diabetic patients: results from the GDMD study in China. Cardiovasc Diabetol. 2018;17(1):84.
Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256(20):2835–8.
Emerging Risk Factors C, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000.
Tg, Hdl Working Group of the Exome Sequencing Project NHL, Blood I, Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371(1):22–31.
Haase CL, Tybjaerg-Hansen A, Qayyum AA, Schou J, Nordestgaard BG, Frikke-Schmidt R. LCAT, HDL cholesterol and ischemic cardiovascular disease: a Mendelian randomization study of HDL cholesterol in 54,500 individuals. J Clin Endocrinol Metab. 2012;97(2):E248–56.
Bjornstad P, Maahs DM, Wadwa RP, Pyle L, Rewers M, Eckel RH, Snell-Bergeon JK. Plasma triglycerides predict incident albuminuria and progression of coronary artery calcification in adults with type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes Study. J Clin Lipidol. 2014;8(6):576–83.
Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16(1):108.
Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, Kim MJ, Kim MK, Park JS. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17(1):41.
Khetarpal SA, Rader DJ. Triglyceride-rich lipoproteins and coronary artery disease risk: new insights from human genetics. Arterioscler Thromb Vasc Biol. 2015;35(2):e3–9.
Johansen CT, Kathiresan S, Hegele RA. Genetic determinants of plasma triglycerides. J Lipid Res. 2011;52(2):189–206.
Jorgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjaerg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34(24):1826–33.
Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45(11):1345–52.
Tseng CH. Lipid abnormalities associated with urinary albumin excretion rate in Taiwanese type 2 diabetic patients. Kidney Int. 2005;67(4):1547–53.
Gall MA, Hougaard P, Borch-Johnsen K, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ. 1997;314(7083):783–8.
Tseng CH. Differential dyslipidemia associated with albuminuria in type 2 diabetic patients in Taiwan. Clin Biochem. 2009;42(10–11):1019–24.
Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–35.
Rutledge JC, Ng KF, Aung HH, Wilson DW. Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat Rev Nephrol. 2010;6(6):361–70.
Wahl P, Ducasa GM, Fornoni A. Systemic and renal lipids in kidney disease development and progression. Am J Physiol Renal Physiol. 2016;310(6):F433–45.
Lee HS. Mechanisms and consequences of hypertriglyceridemia and cellular lipid accumulation in chronic kidney disease and metabolic syndrome. Histol Histopathol. 2011;26(12):1599–610.
Eknoyan G, Hostetter T, Bakris GL, Hebert L, Levey AS, Parving HH, Steffes MW, Toto R. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK). Am J Kidney Dis. 2003;42(4):617–22.
Authors’ contributions
YW analyzed the data and wrote the manuscript. YY performed statistical analysis. AW contributed to acquisition of data. ZG, XT, LY, QW, WW, ZL, GQ, and LC searched data. YM reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank the participants and their families for participating in this study.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used to support this study are not freely available in view of participants’ privacy protection.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the Committee on Human Research at Rui-Jin Hospital affiliated with the School of Medicine, Shanghai Jiao Tong University. Written informed consents were obtained from all participants before data collection.
Funding
Present work was supported by Chinese Society of Endocrinology, the Key Laboratory for Endocrine and Metabolic Diseases of Ministry of Health (1994DP131044), the National Key New Drug Creation and Manufacturing Program of Ministry of Science and Technology (2012ZX09303006-001), the National High Technology Research and Development Program of China (863 Program, 2011AA020107). National Science and Technology Major Project 288 (2011ZX09307-001-08).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Table S1.
Associations between lipid parameters and eGFR in the total subjects, men and women. Table S2. Associations between TG and UACR in pre-diabetic population.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, YX., Wang, AP., Ye, YN. et al. Elevated triglycerides rather than other lipid parameters are associated with increased urinary albumin to creatinine ratio in the general population of China: a report from the REACTION study. Cardiovasc Diabetol 18, 57 (2019). https://doi.org/10.1186/s12933-019-0863-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-019-0863-8