Abstract
Background
Eccrine porocarcinoma is an extremely rare skin adnexal malignant neoplasia with highly invasive and metastatic potential. We report an additional case of eccrine porocarcinoma with intracranial metastases. This case is characterized by a complete record of the progress of eccrine porocarcinoma, its immunohistochemistry after three operations showed a progressive increase in the level of Ki-67 index.
Case presentation
We herein report a case of a 37-year-old-male with eccrine carcinoma occurring on the left posterior occipital scalp which invaded the skull and dura, presenting with progressive headache. This patient has performed three surgeries in total. During the last hospitalization, he underwent an extended surgical resection, lymphadenectomy, myocutaneous flap transplantation and vascular anastomosis in our institution. After surgery, he was treating with radiotherapy at 200 Gray in 12 fractions. But one year after the operation, he developed chest tightness, imaging examination and biopsy puncture revealed pulmonary metastasis.
Conclusion
Intracranial metastasis of eccrine porocarcinoma is a late event with poor prognosis. This case emphases on that progressively increased level of Ki-67 index may predict more chance to occur the intracranial metastasis of scalp eccrine porocarcinoma, long-term follow-up and appropriately dense follow-up interval is necessary.
Similar content being viewed by others
Background
Eccrine porocarcinoma or malignant eccrine carcinoma, is a rare malignant tumor of the skin adnexa with highly invasive and metastatic potential, occurring mainly in the dense regions of eccrine sweat gland, accounting for about 0.005% of all malignant epithelial tumors, presenting approximate morbidity in genders [1,2,3]. The specific etiology of eccrine porocarcinoma is not yet clear, local skin irritation, X-ray irradiation may be its cause. Local repeated recurrence and tumor metastasis are major clinical features of eccrine porocarcinoma [4], tumor tissue usually metastasize to local lymph nodes or adjacent cutaneous, and less common distant organs for metastasis include the breast, liver, lungs, retroperitoneum, ovaries [5]. In general, the recurrence rate and lymph node metastasis rate of eccrine porocarcinoma patients were 20% respectively [6, 7]. At present, the treatment of sweat gland carcinoma (SGC) is still constantly evolving, but its first choice is to take a surgical resection [8]. However, there is no final conclusion about whether radiotherapy or chemotherapy is efficient for sweat gland carcinoma. As review of the literatures revealed, there are only a total of 5 cases about intracranial metastasis and invasion of scalp sweat gland carcinoma were reported to date (Table 1) [3, 9, 10]. We report an additional case of eccrine porocarcinoma with intracranial metastases. Besides, review some existing literatures about this disease and its management.
Case presentation
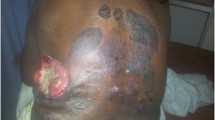
A 37-year-old man developed a gradually growing nodule on the left posterior occipital scalp, which he had first noticed 2 years previously. Excision of scalp mass was performed in local hospital and the postoperative pathology showed skin attachment malignancy. In February 2017, this patient addressed to our hospital for the recurrence of lesion. Brain MRI (Fig. 1. a, b) suggested that soft tissue tumor of the left occipital superior scalp. Then we completed a wide local excision of the posterior occipital region of scalp and grind local skull that has been invaded by tumor. The pathological diagnosis of the mass was eccrine porocarcinoma. No postoperative radiotherapy or chemotherapy was given after two surgery operation.
(a, b) A circular abnormal signal is seen on the left occipital scalp. The border of lesion is clear and its size is about 3.3 × 1.8 cm, the adjacent bone is thin. (c,d) There are enhanced abnormal signals on the left occipital skin, subcutaneous and adjacent meninges. (e,f) Postoperative examination of MRI image showing cranial screw fixation, no recurrence
More than seven months after the operation in our hospital, patient felt pain on the left side of the top occipital area. Head MRI (Fig. 1. c, d) suggested that recurrence and intracranial metastasis of the tumor, and adjacent cranium is involved. PET/CT showed that left occipital area’s FDG metabolism increased, consider tumor recurrence (Fig. 2). Other area showed no abnormal increase in metabolism of FDG. After informed consent, extended surgical resection was taken. We excised the local skull and dura both invaded by tumor. Last, myocutaneous flap transplantation and revascularization was performed (Fig. 3). The pathologic diagnosis was eccrine porocarcinoma (Fig. 4). This patient accepted radiotherapy at 200 Gy in 12 fractions after the surgery and showed no signs of recurrence 6 months after the surgery (Fig. 1. e, f). The positive results of immunohistochemistry after three surgery operation were showed in Table 2. One year after the operation, this patient developed chest tightness, imaging examination and biopsy puncture revealed pulmonary metastasis. And he accepts palliative treatment nowadays.
(a, b) Histopathology revealed cells contained atypical cell nuclei with conspicuous nucleoli, and small amount of eosinophilic cytoplasm. Mitotic figures and apoptotic cells were existed in the lesion (original magnification × 100 or 400, H&E). (c, d, e, f, g, h) Immunohistochemically, the lesional cells were positive when stained with antibodies against cytokeratin 5/6, Ki-67, and PAS, highlighted scattered ductal structures throughout the neoplasm
Discussion and conclusions
Eccrine porocarcinoma is a rare clinical tumor, tends to arise in areas with high concentration of apocrine sweat glands, like the axilla, followed by lower extremities (35%), head and neck (24%) and upper extremities (14%) [2, 11]. Its clinical manifestation is mostly a painless slow-growing subcutaneous solitary mass, but sometimes, tumor may rapidly expand in a short term. Neoplasms usually are normal skin color, maybe light red or purple. And tumor tissue adhered to the skin surface, few cases presented local ulceration [12]. In 1969, Mishima and Marioka formally proposed the term eccrine porocarcinoma [13]. Eccrine porocarcinoma has a high rate of recurrence and metastatic, its mortality rate of metastatic cases increased significantly to 80% with distant metastasis and 65% with lymph nodes [11].
At present, the treatment of eccrine porocarcinoma still follows the principle of sweat gland carcinoma. Mohs micrographic surgery (MMS) or complete circumferential peripheral and deep margin assessment (CCPDMA) are recommended as first-line treatment for patient who is suspected to have sweat gland carcinoma or pathologically confirmed sweat gland carcinoma. Wide local excision can be performed in lieu of MMS and CCPDMA, but the extent of resection should be at least 2 cm from the edge [14]. The resection margin needs to be diagnosed pathologically, or it is necessary to send surgical margin tissue to frozen examination to improve the thoroughness of resection. Due to the fact that primary lesion cannot be directly sutured after resection, skin grafting and partial flap repairing should be given [8, 15].
Status of lymph nodes can be used as an important factor to observe the prognosis of SGC. Lymph node biopsy and resection has clear guiding significance for comprehensive treatment and prognosis of early SGC with regional lymph node metastasis [16, 17]. Another retrospective research performed an analysis of 186 patients: 96% of patients underwent surgical resection or extended resection, 69% of patients underwent lymph node dissection. The median survival time was approximately 51.5 months. Patient’s survival time was significantly shorter who with lymph node metastasis or distant metastasis [18].
The efficacy of radiotherapy and chemotherapy for SGC remains controversial [19]. Some studies pointed out radical radiotherapy could be given after surgery to reduce local recurrence and control distant metastasis of easy-relapsed tumors [20]. In another research, 2 cases of SGC patients with axillary lymph node positive received 50Gy/25f adjuvant radiotherapy after surgery. No recurrence after following up for 9 months and 10 months, respectively [21]. Recent studies found that some patients with hidradenocarcinoma showed positive expression of hormone receptor ER, PR, AR, and HER-2 [22]. Schroder et al. performed tamoxifen treatment in a 64-year-old woman with ER-positive hidradenocarcinoma and achieved complete relief for up to 3 years [23]. These researches suggest in hidradenocarcinoma with positive expression of ER or PR, tamoxifen and trastuzumab can be used as treatment options. And another case of patient with metastatic SGC suggested that the combination of pertuzumabbased targeted therapy with taxane chemotherapy might develop into a new treatment option [24]. In addition, topical Aminolevulinic Acid Photodynamic therapy could be an effective treatment method to improve surgical outcomes of hidradenocarcinoma [25].
In this case, recurrence time of the tumor were fourteen months and ten months, respectively. Because of the diffuse infiltrative growth of sweat gland carcinoma tissue, so surgical resection cannot effectively control the recurrence of sweat gland carcinoma [26]. And the absence of chemotherapy or radiotherapy in previous hospitalizations may connect with its recurrence. It is worth mentioning that immunohistochemistry after three operations showed a progressive increase in the level of Ki-67 index (Table 2). Ki-67 is usually overexpressed in malignant soft tissue tumors and is associating with tumor development, infiltration, metastasis, and prognosis [27]. The high level of Ki-67 index may predict its recurrence and metastasis.
In conclusion, the intracranial metastasis of eccrine porocarcinoma is a late event with a poor prognosis. Summarize experience from this case and previous literature, an extended surgical resection is necessary to improve prognosis. Lymphadenectomy is recommended to perform if there are palpable enlarged lymph nodes in the clinical or imaging evidence indicates local lymph node metastasis. And radiotherapy and chemotherapy can be used to compensate for the disadvantages of insufficient surgical resection. If patient is diagnosed as scalp sweat gland carcinoma with a high level of Ki-67 index, prolonging follow-up time and shortening the follow-up interval is mandatory.
Consent
Written consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Availability of data and materials
All data of this patient of this case report is included in this published article.
Abbreviations
- BCL-2:
-
B cell lymphoma-2
- CCPDMA:
-
Complete circumferential peripheral and deep margin assessment
- CK:
-
Cytokeratin
- CT:
-
Computed tomography
- EMA:
-
Epittielial membrane antigen
- FDG:
-
Fluorodeoxyglucose
- Fig:
-
Figure
- Gy:
-
Gray
- Ki-67:
-
Nuclcar- associated antigen Ki- 67
- MMS:
-
Mohs micrographic surgery
- MRI:
-
Magnetic resonance imaging
- PAS:
-
Periodicacid-schiff
- SGC:
-
Sweat gland carcinoma
References
Melgandi W, Benson R, Hakin A, Bhasker S. Porocarcinoma scalp with high risk features treated with surgery and adjuvant radiotherapy: a case report and review of literature. J Egypt Natl Canc Inst. 2016;28(3):195–8.
Kathrotiya P, Bridge A, Warren S, Do H, Klenk A, Xu L, Mathur A. Primary apocrine adenocarcinoma of the axilla. Cutis. 2015;95(5):271–4.
Khaja. M, Ashraf. U, Mehershahi. S, Ayyadurai. P, Malik. S: Recurrent Metastatic Eccrine Porocarcinoma: A Case Report and Review of the Literature. The American Journal of Case Reports 2019, 20:179–183.
Flux K, Brenn T. Cutaneous sweat gland carcinomas with basaloid differentiation: an update with emphasis on differential diagnoses. Clin Lab Med. 2017;37(3):587–601.
de Bree E, Volalakis E, Tsetis D, Varthalitis Y, Panagiotidis J, Romanos J, Tsiftsis DD. Treatment of advanced malignant eccrine poroma with locoregional chemotherapy. Br J Dermatol. 2005;152(5):1051–5.
Kalogeraki A, Tamiolakis D, Tsagatakis T, Geronatsiou K, Haniotis V, Kafoussi M. Eccrine porocarcinoma: cytologic diagnosis by fine needle aspiration biopsy (FNAB). Acta Medica Port. 2013;26(4):467–70.
Pal S, Phukan J, Bose K, Saha A. Cytodiagnosis of eccrine Porocarcinoma of the scalp. Int J Trichology. 2017;9(4):184–6.
Soni A, Bansal N, Kaushal V, Chauhan AK. Current management approach to hidradenocarcinoma: a comprehensive review of the literature. Ecancermedicalscience. 2015;9:517.
Kim JW, Oh DJ, Kang MS, Lee D, Hwang SW, Park SW. A case of metastatic eccrine porocarcinoma. Acta Derm Venereol. 2007;87(6):550–2.
Waqas O, Faisal M, Haider I, Amjad A, Jamshed A, Hussain R. Retrospective study of rare cutaneous malignant adnexal tumors of the head and neck in a tertiary care cancer hospital: a case series. J Med Case Rep. 2017;11(1):67.
Salih AM, Kakamad FH, Essa RA, Rauf GM, S AM, H MS, Q SR, a HH, a HD, Othman S: Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep 2017, 30:13–16.
Ahn CS, Sangueza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33(1):53–71.
Vaz Salgado MA, Garcia CG, Lopez Martin JA, Guerra E, Benito A, Sepulveda JM, Carrato A. Porocarcinoma: clinical evolution. Dermatol Surg. 2010;36(2):264–7.
Worley B, Owen JL, Barker CA, Behshad R, Bichakjian CK, Bolotin D, Bordeaux JS, Bradshaw S, Cartee TV, Chandra S, et al. Evidence-based clinical practice guidelines for microcystic adnexal carcinoma: informed by a systematic review. JAMA Dermatol. 2019.
Liapakis I, Korkolis D, Koutsoumbi A, Fida A, Kokkalis G, Vassilopoulos P. Malignant hidradenoma: a report of two cases and review of the literature. Anticancer Res. 2006;26(3B):2217–20.
Wauer U, Lorenz D, Sellei R, Zoga E. Braun S: [atypical course of an apocrine sweat gland carcinoma of the axilla : a very rare malignant tumor and its interdisciplinary treatment]. Hautarzt. 2017;68(10):831–4.
Bogner PN, Fullen DR, Lowe L, Paulino A, Biermann JS, Sondak VK, Su LD. Lymphatic mapping and sentinel lymph node biopsy in the detection of early metastasis from sweat gland carcinoma. Cancer. 2003;97(9):2285–9.
Hollowell KL, Agle SC, Zervos EE, Fitzgerald TL. Cutaneous apocrine adenocarcinoma: defining epidemiology, outcomes, and optimal therapy for a rare neoplasm. J Surg Oncol. 2012;105(4):415–9.
Wang X, Wang H, Zheng J, Sui J. Primary cutaneous sweat gland carcinoma. J Cancer Res Ther. 2014;10(2):390–2.
Shiohara J, Koga H, Uhara H, Takata M, Saida T. Eccrine porocarcinoma: clinical and pathological studies of 12 cases. J Dermatol. 2007;34(8):516–22.
Seong MK, Kim EK, Han K, Seol H, Kim HA, Noh WC. Primary apocrine sweat gland carcinomas of the axilla: a report of two cases and a review of the literature. World J Surg Oncol. 2015;13:59.
Gauerke S, Driscoll J. Hidradenocarcinomas: a brief review and future directions. Archives of Pathology & laboratory Medicine. 2010;134(5):781–65.
Schröder U, Dries V, Klussmann J, Wittekindt C, Eckel H. Successful adjuvant tamoxifen therapy for estrogen receptor-positive metastasizing sweat gland adenocarcinoma: need for a clinical trial? Ann Otol Rhinol Laryngol. 2004;113:242–4.
Otsuka M, Yamasaki O, Kaji T, Shien T, Iwatsuki K. Metastatic cutaneous apocrine adenocarcinoma treated with a combination of Pertuzumab-based targeted therapy and Taxane chemotherapy: a case report. JAMA Dermatol. 2016;152(1):111–3.
He X, Yang Y, Yang Y, Wang Y, Wang W, Song Y, Zeng Y, Yang Y, Zhang X, Li G, et al. Treatment of sweat gland carcinoma with topical Aminolevulinic acid photodynamic therapy: an effective treatment method to improve surgical outcomes. Photodiagn Photodyn Ther. 2017;17:233–5.
van der Horst MPJ, Brenn T. Update on malignant sweat gland tumors. Surg Pathol Clin. 2017;10(2):383–97.
Scholzen. T, Gerdes. J: the Ki-67 protein: from the known and the unknown. J Cell Physiol 2000, 182(3):311–322.
Acknowledgements
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
JS and XP contributed to manuscript writing and acquisition of the data. YL and DP contributed to analysis and interpretation of the data. YM contributed to the critical revision of the manuscript for intellectual content. RZ contributed to the critical revision of the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethics approval has been obtained from the ethics committee of First Affiliated Hospital of Zhejiang University. Patient’s consent has been given by this patient.
Consent for publication
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shen, J., Pan, X., Lu, Y. et al. A case of eccrine porocarcinoma characterized by a progressive increase in the level of Ki-67 index: case report and review of literature. BMC Surg 19, 142 (2019). https://doi.org/10.1186/s12893-019-0595-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-019-0595-4