Abstract
Background
Alectinib, a second-generation anaplastic lymphoma kinase (ALK) inhibitor, is a key drug for ALK rearranged lung adenocarcinoma. Interstitial lung disease (ILD) is an important adverse effect of alectinib, which generally requires termination of treatment. However, we treated two patients with drug-induced ILD who continued to receive alectinib.
Case presentation
Patient 1 was a 57-year-old male with an ALK-rearranged Stage IV lung adenocarcinoma who was administered alectinib as first-line therapy. Computed tomography (CT) detected asymptomatic ground-glass opacity (GGO) on day 33 of treatment. Alectinib therapy was therefore discontinued for 7 days and then restarted. GGO disappeared, and the progression of ILD ceased. Patient 2 was a 64-year-old woman with an ALK-positive lung adenocarcinoma who was administered alectinib as third-line therapy. One year later, CT detected GGO; and she had a slight, nonproductive cough. Alectinib therapy was continued in the absence of other symptoms, and GGO slightly diminished after 7 days. Two months later, CT detected increased GGO, and alectinib therapy was continued. GGO diminished again after 7 days. The patient has taken alectinib for more than 2 years without progression of ILD.
Conclusions
Certain patients with alectinib-induced ILD Grade 2 or less may continue alectinib therapy if they are closely managed.
Similar content being viewed by others
Background
Alectinib is one of the key drugs for treating patients with ALK-positive non-small cell lung cancer (NSCLC), because it is effective and is well tolerated [1]. Interstitial lung disease (ILD) is an important adverse effect associated with alectinib treatment as well as with all tyrosine kinase inhibitors (TKI) [2]. Generally, when ILD occurs, TKI therapy should be terminated, although treatment with certain molecular targeting inhibitors such as those specific for mammalian target of rapamycin (mTOR) can be continued [3]. It is unclear whether the physician should continue alectinib when ILD is diagnosed. Here we report two patients with alectinib-induced ILD who were able to continue alectinib therapy.
Case presentation
Patient 1
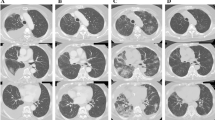
A 57-year-old male smoker with lung adenocarcinoma of his right lower lobe was positive for EML4-ALK and positive for ALK. Metastases were present at the third left rib and bilateral adrenal glands, leading to a diagnosis of Stage IV disease. Alectinib (300 mg twice daily) was administered as first-line treatment as per standard of care. CT revealed that the sizes of the primary lesion and other metastases were much smaller compared with baseline. However, 33 days after alectinib administration, CT revealed patchy ground glass opacity (GGO) of the left lower lobe (Fig. 1a). We suspected alectinib-induced ILD, and discontinued treatment. Laboratory data did not detect significant inflammation. Bronchoalveolar lavage fluid (BALF) analysis of the left B9 revealed to be lymphocyte-predominant for 54%. The results of blood tests and microbial culture of the BALF did not indicate any infection (Table 1), so we diagnosed alectinib-induced ILD. But we did not use corticosteroid because he had no symptom of ILD.Alectinib was therefore reintroduced on day 40with careful observation. The GGO in the left lower lobe disappeared on day 61 (Fig. 1b). The patient did not show any symptoms after a 7-month course of alectinib.
Patient 2
A 64-year-old woman nonsmoker had experienced relapsing adenocarcinoma 19 months after undergoing lung surgery and postoperative chemotherapy. She was positive for EML4-ALK. Crizotinib therapy was started as first-line treatment. However, multiple lung and brain metastases and carcinomatous lymphagitis developed after 5 months. Whole brain radiotherapy and two cycles of pemetrexed, bevacizumab maintenance therapy after 4 cycles of carboplatin-pemetrexed-bevacizumab therapy were administered. However, 6 months later, brain MRI revealed tumor dissemination to the meninges. Therefore, she received 300-mg alectinib twice daily. Twelve months later, a chest CT revealed GGO in the left lower lobe (Fig. 2a). She presented with a slight, nonproductive cough, no fever or dyspnea, and her blood values were within their normal ranges. Alectinib-induced ILD Grade 2 (Common Terminology Critera for Adverse Events version 4.0) was suspected. Since her symptom was only cough and very slight, we had continued alectinib with careful observation, not using corticosteroid. After 7 days, her cough and the GGO had been improved spontaneously (Fig. 2b), so alectinib treatment was continued. But it became more pronounced 2 months later (Fig. 2c). Then she did not present cough and any other symptoms or abnormal laboratory values. Blood tests and microbial culture of BALF did not detect any infection and cell count of BALF was normal (Table 1). There was no other cause of the GGO, so we diagnosed alectinib-induced ILD. But she was not considered to have significant symptom caused by ILD, we therefore continued alectinib treatment and found that the GGO diminished after 1 week. (Fig. 2d). She has taken alectinib for 2 years without progression of ILD.
Discussion and conclusions
Alectinib is a key drug for treating patients with EML4-ALK-positive NSCLC. In particular, alectinib is highly effective and well tolerated [1], although some patients may experience severe acute ILD [4]. In the ALEX trial and the J-ALEX trial, the incidence of alectinib-induced ILD was reported 1% and 8%, respectively [5, 6]. Physicians generally must discontinue alectinib in such cases, although we believe that certain patients can continue therapy.
In general, drug-induced ILD is suspected when the following criteria are met:(1) history of exposure to the suspected drug, (2) report of suspected previous drug-induced ILD, (3) exclusion of other causes, (4) improvement after discontinuation of a suspected drug, and (5) recurrence of symptoms on rechallenge [7]. Patient 1 did not meet criteria 5 and patient 2 did not meet criteria 4 and 5.; however, there was no evidence of infection and other etiologies of ILD. Furthermore, Ikeda et al. reported a patient with alectinib-induced ILD who had GGO and no clinical symptoms, similar to our patients [8]. We conclude therefore that our patients had alectinib-induced ILD.
Generally, drug-induced ILD is characterized by lymphocyte-predominant BALF, while bacterial pneumonia may be associated with neutrophil-predominant BALF. Ait-Tahar et al. reported higher immune responses of B and cytotoxic T cells in ALK-positive patients with anaplastic large cell lymphoma than in ALK-negative patients [9]. It is suggested that having ALK fusion gene may lead to higher immune response. Analysis of the BALF of Patient 1 revealed lymphocyte-predominant disease, which might reflect “ALK fusion gene-related drug hypersensitivity”. This “hypersensivity” may lead to the risk of ILD. Otherwise the BALF of Patient 2 was neutrophil-predominant, which may be influenced by the low recovery rate from BALF. Additionally, since there was no evidence of other etiology of the GGO, the absence of lymphocyte-predominant BALF did not exclude drug-induced ILD.
We continued alectinib despite the suspicion of ILD, because patient1 did not have any symptoms, patient 2 had only very sligh cough. Créquit P et al. reported that six of 29 patients with ILD who were treated with crizotinib, the first available ALK inhibitor for treating NSCLC, had few clinical symptoms [10]. Moreover, they reported that patients with crizotinib-induced ILD had longer median progression-free survival compared with those without crizotinib-ILD (19.9 vs 6.2 months, p = 0.04). Therefore, these findings suggest that a patient with ALK inhibitor-induced ILD may exhibit a higher response compared with those without ALK inhibitor-induced ILD. To our knowledge, this is the first report that patients with ILD Grade 2 or less were able to continue alectinib therapy.
Alectinib may induce severe ILD. For example, CT detected bilateral GGO in a patient with progressive dyspnea [4]. According to Créquit P et al., a patient with crizotinib-induced ILD died from acute respiratory distress syndrome on day 28 after the initiation of crizotinib therapy, and this patient previously experienced reversible erlotinib-induced ILD [10]. Therefore, if physicians want to continue alectinib treatment for a patient with alectinib-ILD Grade2 or less, the very careful observation is needed.
There are some limitations to the present study. First, we retrospectively studied two patients who were treated at a single center, and more patients must be studied, or a multicenter prospective study will be required. Second, the recovery rate of BALF was insufficient, so BALF analysis may not have reflected the effect of alectinib for the lungs accurately. Third, it is not clear if our patients responded longer to therapy compared with those without alectinib-induced ILD.
We conclude that certain patients with alectinib-induced ILD Grade2 or less may continue alectinib therapy if they are closely managed, because they may therefore achieve a prolonged response to therapy that leads to longer survival.
Abbreviations
- ALK:
-
Anaplastic lymphoma kinase
- BALF:
-
Bronchoalveolar lavage fluid
- CT:
-
Computed tomography
- GGO:
-
Ground-glass opacity
- ILD:
-
Interstitial lung disease
- mTOR:
-
Mammalian target of rapamycin
- NSCLC:
-
Non-small cell lung cancer
- TKI:
-
Tyrosine kinase inhibitor
References
Seto T, Kiura K, Nishio M, Nakagawa K, Maemondo M, Inoue A, Hida T, Yamamoto N, Yoshioka H, Harada M, Ohe Y, Nogami N, Takeuchi K, Shimada T, Tanaka T, Tamura T. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol. 2013;14:590–8.
Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: Gefitinib (ZD1839) (Iressa®) tablets. Oncologist. 2003;8:303–6.
White D, Camus P, Endo M, Escudier B, Calvo E, Akaza H, Uemura H, Kpamegan E, Kay A, Robson M, Ravaud A, Motzer RJ. Noninfectious Pneumonitis after Everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med. 2010;182:396–403.
Yamamoto Y, Okamoto I, Otsubo K, Iwama E, Hamada N, Harada T, Takayama K, Nakanishi Y. Severe acute interstitial lung disease in a patient with anaplastic lymphoma kinase rearrangement-positive non-small cell lung cancer treated with alectinib. Investig New Drugs. 2015;33:1148–50.
Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, Ou SHI, Pérol M, Dziadziuszko R, Rosell R, Zeaiter A, Mitry E, Golding S, Balas B, Noe J, Morcos PN, Mok T. Alectinib versus Crizotinib in untreated ALK-positive non.Small-cell lung cancer. N Engl J Med. 2017;(6) 10.1056/NEJMoa1704795.
Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, Takiguchi Y, Nishio M, Yoshioka H, Imamura F, Hotta K, Watanabe S, Goto K, Satouchi M, Kozuki T, Shukuya T, Nakagawa K, Mitsudomi T, Yamamoto N, Asakawa T, Asabe R, Tanaka T, Tamura T. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39.
Schwaiblmair M, Behr W, Haeckel T, Markl B, Foerg W, Berghaus T. Drug induced interstitial lung disease. Open Respir Med J. 2012;6:63–74.
Ikeda S, Yoshioka H, Arita M, Sakai T, Sone N, Nishiyama A, Niwa T, Hotta M, Tanaka T, Ishida T. Interstitial lung disease induced by alectinib (CH5424802 /RO5424802). Jpn J Clin Oncol. 2015;45:221–4.
Ait-Tahar K, Cerundolo V, Banham AH, Hatton C, Blanchard T, Kusec R, Becker M, Smith GL, Pulford K. B and CTL responses to the ALK protein in patients with ALK-positive ALCL. Int J Cancer. 2006;118:688–95.
P. Créquit, M. Wislez, J. Fleury Feith, N. Rozensztajn, L. Jabot, S. Friard S, A. Lavole, V. Gounant, J. Fillon, M. Antoine, J. Cadranel J. Crizotinib associated with ground-glass opacity predominant pattern interstitial lung disease. A retrospective observational cohort study with a systematic literature review. J Thorac Oncol. 10 (2015) 1148-1155.
Acknowledgments
I am deeply grateful to all the people who were associated with our medical practice.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
All data are available in the manuscript.
Author information
Authors and Affiliations
Contributions
TN wrote the initial draft of the manuscript. YS contributed to collect data, and assisted in the preparation of the manuscript. All other authors have contributed to review the manuscript. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We have obtained written consent from the patient to publish this case report and no ethical committee approval was necessary for this case study.
Consent for publication
Written informed consent in the form of our institution was obtained from the each patients for publication of this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Nitawaki, T., Sakata, Y., Kawamura, K. et al. Case report: continued treatment with alectinib is possible for patients with lung adenocarcinoma with drug-induced interstitial lung disease. BMC Pulm Med 17, 173 (2017). https://doi.org/10.1186/s12890-017-0519-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-017-0519-y