Abstract
Background
Laparoscopy has many advantages when used to assist surgery. However, pneumothorax, as a rare but potentially life-threatening complication, it requires rapid recognition and treatment. CO2 pneumothorax may be distinct from air pneumothorax. Here we present a case with unexpected large and symptomatic CO2 pneumothorax and treated successfully in a conservative way.
Case presentation
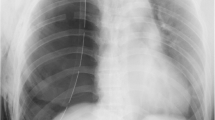
A 27-year-old woman who was scheduled a laparoscopic partial nephrectomy received general anesthesia. At the end of surgery, she waked up and got spontaneous breathing. However, she developed a sudden fall in SpO2 (approximately 30%) and blood pressure with subsequent unconsciousness after switching mechanical ventilation to spontaneous mode. With immediate manual ventilation, SpO2 and blood pressure recovered simultaneously and the patient regained consciousness. Point-of-care chest X-ray revealed a large, right pneumothorax occupying 70% of the hemi-thorax. Without chest drainage, she was extubated in the operating room and treated with supplemental facial mask oxygen therapy in PACU. On the postoperative 5th day, she was discharged without any further complication.
Conclusion
Retroperitoneal laparoscopic surgeries are likely to bring about severe capno-thorax, which could be absorbed rapidly. Chest X-ray could be used to assist diagnosis but point-of-care transthoracic ultrasound is recommended. Even severe capno-thorax could be treated conservatively. This case highlights the awareness and therapeutic choice of noninvasive management for capno-thorax.
Similar content being viewed by others
Background
Laparoscopic surgeries have been widely performed because they have so many advantages as less invasiveness, less postoperative pain, shorter hospital stay and better cosmesis [1]. However, they may also cause severe complications like pneumothorax, pneumomediastinum and subcutaneous emphysema [2]. Laparoscopic partial nephrectomy, which could be performed through insufflating carbon dioxide (CO2) in either the retroperitoneal or peritoneal space to achieve a better view, has been widely used for surgical treatment for T1 stage renal tumor. Pneumothorax is a recognized complication of laparoscopic surgery [3, 4]. Although the incidence of pneumothorax in laparoscopic renal surgery is less than 1%, it’s potentially serious and life-threatening [5], requiring rapid recognition and treatment. For patients with large pneumothorax or obvious symptoms, the common practice is to place a chest tube for drainage. However, CO2 pneumothorax is distinct from air pneumothorax in that CO2 is highly soluble in blood and could be absorbed rapidly [6, 7]. Here, we present a case with an unexpected large volume of CO2 pneumothorax and severe symptoms, which was managed conservatively.
Case presentation
A 27-year-old woman (height 165 cm; weight 92 kg) was admitted to hospital for management of a 1.8 cm × 1.3 cm × 1.7 cm renal mass in the lower pole of the right kidney (examined by MRI) and scheduled for laparoscopic partial nephrectomy under general anesthesia. The patient mentioned she had no other medical history but congenital asymptomatic platybasia. The laboratory examinations were normal except uric acid 425 μmol/L (the normal range is 90–360 μmol/L). There was no abnormity for electrocardiogram (ECG) or chest X-ray.
On the operating day, the patient entered the operating room without premedication. ECG, SpO2, end-tidal carbon dioxide pressure (PetCO2) and bispectral index (BIS) were monitored. A 20G catheter was inserted into her left radial artery to ensure real-time blood pressure monitoring. Anesthesia was induced with remifentanil (target-controlled infusion at effect-site concentration of 3 ng/mL), 150 mg propofol and 50 mg rocuronium. A 7 mm ID endotracheal tube was intubated with an insertion distance of 21 cm at the incisors. The patient was ventilated with volume controlled ventilation mode (setting tidal volume at 500 ml, respiratory rate at 12 times/min, inspiration and expiration ratio at 1:2) and was placed in the left lateral decubitus position. Before pneumoperitoneum, airway pressure was controlled within 20 cmH2O and PetCO2 was controlled between 31 and 35 mmHg. Anesthesia was maintained with intravenous remifentanil (target-controlled infusion at effect-site level of 2–3 ng/mL), propofol (constant infusion), and 60% nitrous oxide balanced with oxygen. Sufentanil (totally 20 μg) and cisatracurium (totally 2 mg) was intermittently injected intravenously and the infusion speed of propofol was adjusted according to BIS within the range of 40 to 60.
The procedure was uneventful though there was an episode. After finishing the first trocar portal, a balloon, made from a sutured latex glove, was used to inflate and dilate retroperitoneum cavity. But the balloon was ruptured during inflation and the suture on the balloon was left in retroperitoneum space. Finally, the suture was found and removed after a period of searching with the help of laparoscopy. Procedure was performed via 3 successfully established trocar portals. The retroperitoneal space was hydrostatically dilated and CO2 was insufflated to a pressure of 14 mmHg. During the procedure, the airway pressure increased to 30 cmH2O and PetCO2 was elevated to 41 mmHg. SpO2 remained 100%. We adjusted tidal volume to 550 ml and respiratory rate to 13 times/min. At the end of the surgery, the retroperitoneal carbon dioxide was retreated and airway pressure decreased to 23–24 cmH2O. Operative duration was almost 90 min, during which the hemodynamic parameters were stable. We stopped all intravenous anesthetics and changed the gas supply to 100% O2 with a flow rate of 5 L/min. The patient woke up quickly and could respond to instructions. Then the patient was placed to supine position. Mechanical ventilation was ceased and the patient had spontaneous breathing. Antagonists of muscle relaxant (neostigmine 2 mg plus atropine 1 mg) were given. Two minutes after the stop of mechanical ventilation, SpO2 decreased rapidly to 30% and blood pressure decreased from 120/79 mmHg to 93/65 mmHg. The patient was unconscious. We conducted manual ventilation immediately and then SpO2 returned to 85–90%. Meanwhile, blood pressure recovered. Immediate auscultation showed decreased breath sounds on the right side of chest and the left side was normal. Immediate arterial blood gas analysis showed PCO2 75 mmHg, PO2 83 mmHg. We woke up the patient and she could breathe spontaneously with better tidal volume. We extubated her endotracheal tube and provided oxygen via facemask. She was able to breathe without distress but felt right chest pain. SpO2 climbed to 91–93% gradually. Point-of-care chest X-ray was performed, demonstrating a large, right pneumothorax occupying 70% of the hemithorax (Fig. 1).
The patient was transferred to post-anesthesia care unit and stayed for 1 h with facemask oxygen inhalation (oxygen flow rate 5 L/min). Her vital signs were normal during the period and SpO2 returned to 100%. She felt right chest pain relieved a bit. Arterial blood gas was re-examined and all parameters were in normal range. The patient was later sent back to general ward.
In general ward, respiratory and hemodynamic parameters of the patient remained stable during hospital stay. Chest X-ray taken on the first post-operative day revealed complete re-expansion of the right lung (Fig. 2). She claimed no chest pain and no other symptoms. The patient was discharged home on postoperative day 5.
Discussion and conclusion
Large or symptomatic pneumothorax usually necessitates thoracocentesis or chest tube placement. But capno-thorax may warrant a different treatment. Table 1 summarized cases which reported moderate to large symptomatic capno-thorax while implemented conservative treatments. Different from these described above, in our case, the type of surgery route was retroperitoneal. The symptom was more severe (SpO2 30%) and happened after gas deflation and before extubation. The patient was awake at first but fell into unconsciousness later. It’s more urgent and hard for us to diagnose and decide whether it was suitable to adopt an invasive method. Fortunately, the severe condition didn’t last for a long time. We also retrieved some cases in whom capno-thorax occurred in retroperitoneal urological surgeries. But most of these happened intraoperatively and implemented thoracocentesis or chest tube placement [8,9,10,11], or had confirmed diaphragm injury and recovered after converting to open surgery [12]. Although study showed CO2 absorption during laparoscopy did not depend on the route of surgery [13, 14], for retroperitoneal route, the restriction on working space and field of view may increase the risk of inadvertent organ damage.
Causes for pneumothorax
Pneumothorax during laparoscopic procedures has been reported and the possible causes are [3, 8,9,10,11,12,13,14]: First, barotrauma and rupture of bullae during mechanical ventilation or during central venous line placement may contribute to air pneumothorax. Second, unrecognized congenital defect such as diaphragmatic defects [15] or pleuroperitoneal fistulas could predispose patients to pneumothorax. Third, increased operative duration is likely to compel more CO2 absorption. Study showed that PetCO2 greater than 50 mmHg and operative time greater than 200 min were risk factors for pneumothorax [16]. Fourth, higher insufflation pressure likely leads to a large amount of CO2 absorption. Several cases have been reported to get pneumothorax during laparoscopic surgeries with an insufflation pressure of 12–15 mmHg [2, 17, 18] whereas insufflation pressure under 12 mmHg might still cause pneumothorax [19]; furthermore, rapid insufflation would allow the intracavitary pressure to increase suddenly, contributing to pneumothorax [11] because retroperitoneal space is not a true cavity and has lower compliance than the abdominal cavity. Fifth, it is also possible for CO2 to dissect into the pleural space along the vena cava or aorta [16]. Finally, pleural injury due to errant trocar placement or diaphragmatic injury possibly accounts for pneumothorax [4, 20].
In our case, the symptomatic pneumothorax was unanticipated as the procedure and anesthesia practice was uneventful. The patient was young and healthy. Operating duration and intraoperative PetCO2 were also acceptable. But the diaphragmatic defect cannot be excluded. Though operation on the lower pole of the kidney is less likely to injure diaphragm, the procedure to explore the lost suture might injure it unconsciously. Besides, CO2 could also dissect into the pleural space along the vena cava. All these possible reasons suggest not air but CO2 pneumothorax.
Clinical manifestations and diagnosis
Pneumothorax could bring out pulmonary atelectasis, elevate inspiratory airway pressure, PetCO2 as well as PaCO2 and decrease breath sounds and blood pressure. Hypotension occurs due to the decreased venous return and cardiac output. However, in this case, these changes were not obvious during procedure but appeared after the termination of mechanical ventilation. Possible reasons are that respiratory depression after the cessation of mechanical ventilation combined with pneumothorax exaggerated the clinical manifestation.
Chest computer tomography scan is the gold standard for diagnosis of pneumothorax [21, 22]. Chest X-ray is usually as the initial tool to detect the potential cases. All the cases list above was diagnosed by Chest X-ray. However, it has been demonstrated that point-of-care transthoracic ultrasound, a cheaper, nonradiative and timely tool, is more accurate than chest X-ray with 81% sensitivity and 100% specificity [23, 24]. Anterior absent lung sliding plus A lines plus lung point indicated pneumothorax [23]. If lung is totally collapsed, lung point isn’t visualized; lung point on the mid axillary line differentiates large and small pneumothorax, coinciding with a cut-off set at 15% of lung collapse [25], and could guide decision-making in treatments. This technique is extremely important for anesthesiologists to practice in the operating room. However, we didn’t perform ultrasound scan because of inexperience.
Practice taken to alleviate carbon dioxide pneumothorax
The treatment can vary depending on the causes and severity of the pneumothorax. FiO2 should be increased and N2O supply should be stopped. CO2 insufflation could be reduced or discontinued. Endotracheal intubation, hyperventilation and higher positive end-expiratory pressure should be maintained [26].
Traditionally, a chest tube could be placed if a large pneumothorax or consequent cardiovascular or respiratory collapse is diagnosed. But capno-thorax may warrant a different treatment strategy. The solubility of CO2 is 20 times more than nitrogen and has an increased diffusion coefficient compared to air, which is composed mostly of nitrogen and oxygen. Higher solubility of a gas means more molecules can diffuse across a membrane in a given time. Experimental and clinical evidence demonstrated that resolution of capno-thorax can be more rapid than air pneumothorax, and usually complete re-expansion of the compressed lung could be achieved within several hours [15, 27,28,29], even within 30–60 min [30].We didn’t implement thoracentesis or place a chest tube but provided oxygen supply and intensive care because it is found that the probability of pneumothorax caused by CO2 is higher than that by air, patient’s condition was getting better and the source of CO2 was no longer feeding the retroperitoneum. The woman returned to normal state within 1 h although chest pain disappeared completely the next day.
In conclusion, pneumothorax, although rare, is a serious complication of laparoscopic nephrectomy. Anesthesiologists, when facing this crisis, should recognize pneumothorax right away. Point-of-care transthoracic ultrasound should be recommended to diagnose. Different from air pneumothorax, CO2 pneumothorax, even with a large volume, can be resolved spontaneously without invasive management.
Abbreviations
- BIS:
-
bispectral index
- CO2 :
-
carbon dioxide
- CXR:
-
chest X-ray
- ECG:
-
electrocardiogram
- FiO2 :
-
fraction of inspired oxygen
- MRI:
-
magnetic resonance imaging
- N2O:
-
nitrous oxide
- O2 :
-
oxygen
- PCO2 :
-
carbon dioxide partial pressure
- PetCO2 :
-
end-tidal carbon dioxide pressure
- PO2 :
-
oxygen partial pressure
- SpO2 :
-
pulse oxygen saturation
References
Hamilton BD, Gatti JM, Cartwright PC, Snow BW. Comparison of laparoscopic versus open nephrectomy in the pediatric population. J Urol. 2000;163(3):937–9.
Mamić I, Danolić D, Puljiz M, et al. Pneumothorax and pneumomediastinum as a rare complication of laparoscopic surgery. Acta Clin Croat. 2016;55(3):501–4.
Waterman BJ, Robinson BC, Snow BW, et al. Pneumothorax in pediatric patients after urological laparoscopic surgery: experience with 4 patients. J Urol. 2004;171(3):1256–9.
Inderbir S, Kavoussi LR, Clayman RV, et al. Complications of laparoscopic nephrectomy in 185 patients: a multi-institutional review. J Urol. 1995;154(2):479–83.
Del Pizzo JJ, Jacobs SC, Bishoff JT, Kavoussi LR, Jattett TW. Pleural injury during laparoscopic renal surgery: early recognition and management. J Urol. 2003;169:41–4.
PT Chui T. Gin and SCS Chung. Subcutaneous emphysema, pneumomediastinum and pneumothorax complicating laparoscopic vagotomy. Anaesthesia. 1993;48:978–81.
Heddle RM, Platt AJ. Tension pneumothorax during laparoscopic cholecystectomy. Br J Surg. 1992;79:374.
Kusaka J, Goto K, Yamamoto S. Pneumothorax during retroperitoneoscopic nephrectomy: a case report. Masui. 2004;53(12):1411–3.
Matsushita Y, Miyake H, Motoyama D. Contralateral pneumothorax during retroperitoneal laparoscopic donor nephrectomy: a case report. Asian J Endosc Surg. 2017;10(2):202–4.
Stern JA, Nadler RB. Pneumothorax masked by subcutaneous emphysema after laparoscopic nephrectomy. J Endourol. 2004;18(5):457.
Shanberg AM, Zagnoev M, Clougherty TP. Tension pneumothorax caused by the argon beam coagulator during laparoscopic partial nephrectomy. J Urol. 2002;168(5):2162.
Ozasa H, Uchida H, Moto-Oka A. Intraoperative pneumothrax during retroperitoneal laparoscopic surgery. J Anesth. 2004;18(4):318–9.
Kadam PG, Marda M, Shah VR. Carbon dioxide absorption during laparoscopic donor nephrectomy: a comparison between retroperitoneal and transperitoneal approaches. Transplant Proc. 2008;40(4):1119–21.
Ng CS, Gill IS, Sung GT, Whalley DG, Graham R, Schweizer D. Retroperitoneoscopic surgery is not associated with increased carbon dioxide absorption. J Urol. 1999;162(4):1268–72.
Park HJ, Kim DK, Yang MK. Carbon dioxide pneumothorax occurring during laparoscopy-assisted gastrectomy due to a congenital diaphragmatic defect: a case report. Korean J Anesthesiol. 2016;69(1):88–92.
Murdock CM, Wolff AJ, Van Geem T. Risk factors for hypercarbia, subcutaneous emphysema, pneumothorax, and pneumomediastinum during laparoscopy. Obstet Gynecol. 2000;95(5):704–9.
Ferzli GS, Kiel T, Hurwitz JB, Davidson P, Piperno B, Fiorillo MA, et al. Pneumothorax as a complication of laparoscopic inguinal hernia repair. Surg Endosc. 1997;11(2):152.
Aravind A, Shroff P, Dewoolkar L. Epigastric pain caused by pneumothorax following extubation in a case of transperitoneal laparoscopic nephrectomy. Intern J Anesthesiol. 2006;13(2). http://ispub.com/IJA/13/2/10355#.
Hagopian EJ, Steichen FM, Lee KF, Earle DB. Gas extravasation complicating laparoscopic extraperitoneal inguinal hernia repair. Surg Endosc. 2001;15:324.
Potter SR, Kavoussi LR, Jackman SV. Management of diaphragmatic injury during laparoscopic nephrectomy. J Urol. 2001;165(4):1203–4.
Danielsen M, Højgaard L, Kjær A, Fischer BM. Positron emission tomography in the follow-up of cutaneous malignant melanoma patients: a systematic review. Am J Nucl Med Mol Imaging. 2014;4(1):17–28.
Schulze C, Hoppe H, Schweitzer W, Schwendener N, Grabherr S, Jackowski C. Rib fractures at postmortem computed tomography (PMCT) validated against the autopsy. Forensic Sci Int. 2013;233(1):90–8.
Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure*: the BLUE protocol. Chest. 2008;134(1):117–25.
Nagarsheth K, Kurek S. Ultrasound detection of pneumothorax compared with chest X-ray and computed tomography scan. Am Surg. 2011;77(4):480–4.
Volpicelli G, Boero E, Sverzellati N, Cardinale L, Busso M, Boccuzzi F. Semi-quantification of pneumothorax volume by lung ultrasound. Intensive Care Med. 2014;40(10):1460–7.
Joris JL, Chiche JD, Lamy ML. Pneumothorax during laparoscopic fundoplication: diagnosis and treatment with positive end-expiratory pressure. Anesth Analg. 1995;81(5):993–1000.
Ludemann R, Krysztopik R, Jamieson GG, Watson DI. Pneumothorax during laparoscopy. Surg Endosc. 2003;17(12):1985–9.
Msezane LP, Zorn KC, Gofrit ON, et al. Case report: conservative management of a large capnothorax following laparoscopic renal surgery. J Endourol. 2007;21(12):1445–8.
Karayiannakis AJ, Anagnostoulis S, Michailidis K, Vogiatzaki T, Polychronidis A, Simopoulos C. Spontaneous resolution of massive right-sided pneumothorax occurring during laparoscopic cholecystectomy. Surg Endosc. 2005;15(2):100–3.
Togal T, Gulhas N, Cicek M, Teksan H, Ersoy O. Carbon dioxide pneumothorax during laparoscopic surgery. Surg Endosc. 2002;16:1242.
Mehran A, Brasesco O, De VE, Szomstein S, Rosenthal R. Intra-operative pneumothorax complicating laparoscopic roux-en-y gastric bypass. Obes Surg. 2004;14(1):124–8.
Altarac S, Janetschek G, Eder E, Bartsch G. Pneumothorax complicating laparoscopic ureterolysis. J Laparoendosc Surg. 1996;6(3):193–6.
Kumar G, Singh A. Pneumothorax during laparoscopic cholecystectomy. Med J Armed Forces India. 2007;63(3):277–8.
Bala V, Kaur MD, Gupta N, Pawar M, Sood R. Pneumothorax during laparoscopic cholecystectomy: a rare but fatal complication. Saudi J Anaesth. 2011;5(2):238–9.
Phillips S, Falk GL. Surgical tension pneumothorax during laparoscopic repair of massive hiatus hernia: a different situation requiring different management. Anaesth Intensive Care. 2011;39(6):1120.
Acknowledgements
Not applicable.
Funding
This work was without financial support.
Availability of data and materials
All data and materials described in the manuscript will be freely available for non-commercial purposes.
Author information
Authors and Affiliations
Contributions
WQF were responsible for obtaining the patient’s informed consent, collecting the data of the patient, follow up, and preparation of the manuscript; ZH participated in the anesthesia and care of the patient and was responsible for revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wu, Q., Zhang, H. Carbon dioxide pneumothorax following retroperitoneal laparoscopic partial nephrectomy: a case report and literature review. BMC Anesthesiol 18, 202 (2018). https://doi.org/10.1186/s12871-018-0662-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-018-0662-x