Abstract
Heat treatment was an eco-friendly modification technology for rubberwood, without the addition of chemical reagents into the wood and wood performance has been improved remarkably. Many effluents are generated from heat-treated rubberwood process, which were rarely researched. The effluents contain relatively high content of volatile organic compounds, which may seriously threaten the environment and human health. In this study, effluents condensated during different heat-treated stages (125 ℃, 145 ℃, 175 ℃, 185 ℃, 215 ℃) and frequently used industrial heat treatment production technology (185 ℃/3 h, 215 ℃/3 h) were characterized by gas chromatography–mass spectrometry (GC–MS). The effluents were classified as aldehydes, phenols, esters, ketones, acids, alcohols, alkanes, anhydride, aromatics and compounds released the most during all heat-treated stages were aldehydes. With the temperature increased, the relative amounts of aldehydes decreased, while the ketones, esters and aromatics increased under acidic constituents (pH ranged from 4.17 to 2.47) and high moisture condition. The vanillin and coniferyl aldehyde accounted for much higher proportion in the aldehydes. The mass loss of rubberwood was larger under 215 ℃/3 h (16.61%), correspondingly the effluents had more kinds of compounds at 215 ℃. These results would provide guidance for research of effluent disposal and transform biomass residues into valuable things.
Similar content being viewed by others
Introduction
In China, the rubber (Hevea brasiliensis) plantations are mainly located in Hainan, Yunnan, and Guangdong provinces. The rubber trees, which provide not only raw materials for the rubber industry but also abundant wood resources, are the main plantation tree species of the tropics [1]. In 2009, the total planted area of rubber trees was 13.314 million hectares in the world’s rubber producing countries. Among them, 12.2251 million hectares were in Asia, accounting for about 90% of the global rubber tree planting area. The rubber tree planted area of China ranked the third, with a total area of 1.161 million hectares [2], and the rubber logs were updated more than 2 million cubic meters annually in China. The main problem associated with using rubberwood is its dimensional instability due to moisture adsorption/desorption [3]. Because of the weak mechanical strength and dimensional instability, rubberwood is usually used to make furniture, which is generally limited to indoor applications. The modification of the rubberwood has been found to be effective in extending the service life and field of rubberwood [4]. Therefore, it is necessary to adopt some methods to improve the value of rubberwood.
Heat treatment was an eco-friendly modification technology, without chemical reagents were added into the wood. Heat treatment improved various wood properties, including the dimensional stability, color, and decay resistance [5]. After the rubberwood was heat treated, the dimensional stability and decay resistance of rubberwood were elevated [6]. However, due to the extracts and structural materials in rubberwood, it can easily release volatile organic compounds (VOCs) and effluents into the environment during the heat-treatment processing. Over time, the VOCs can corrode the wall on the top of house in factory. Currently, there were already some reports on the physical and chemical properties of heat-treated wood, technological parameters of heat treatment [7], although it was short of the study of VOCs emitted from heat-treatment processing.
The VOCs are an important class of air pollutants commonly found in the atmosphere at ground level in all urban and industrial centers [8, 9]. And they usually emitted unpleasant odors and may threaten people’s health and affected their lives [10]. Its emissions caused lots of environmental problems and people’s health problems, such as stratospheric ozone depletion, photochemical ozone formation at ground level, toxic or carcinogenic effects on human health, intensification of global greenhouse effect, accumulation and persistence in the environment [11]. Some study on the VOCs emitted from heat-treated wood has been reported [12,13,14], since heat-treated wood materials and products are used indoors, safety and impact of these new materials on the indoor air quality should be determined. Chu et al. [15] analyzed the VOC emissions and antimicrobial activities of its condensate produced from the production of heat-treated poplar wood and discovered that the concentrations of phenols and ketones in the liquid byproducts decreased when the treatment temperature was increased, the condensate collected at 160 ℃ exhibited considerable biological activity in the bacterial and mildew resistance tests.
Although there are no reports of effluents emitted from heat-treated rubberwood processing, which contains large quantities of sugar, starch and extracts [16], greater quantities of effluents were released during heat-treatment processing. Now the heat-treated rubberwood is an important wood resource for making furniture for export and for the production of panel products, such as particleboard, medium density fiberboard (MDF), wood fiber cement-bonded particleboard, and plywood [17]. It’s necessary to investigate the effluents emitted from the heat-treatment processing of rubberwood. The aim of our work is the characterization of these emissions by gas chromatography–mass spectrometry (GC–MS), and focus on the condensable effluents emitted by rubberwood heat-treatment processing. It would help to speculate, if the effluents may cause discomfort [18] and if they contained valuable products, which are sufficient for an economically viable recovery [19,20,21]. The results will also be useful for the collection and application of the byproducts of the heat-treated wood production industry.
Materials and methods
Heat treatment of rubberwood
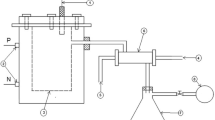
Air dried rubberwood blocks (600 mm × 100 mm × 20 mm) were collected from a rubber tree of rubber clone PR107 (25 years old) at Team 5 of the Experimental Farm of Chinese Academy of Tropical Agricultural Science, in Danzhou, from the Hainan province in China. The rubberwood blocks, whose size was similar with the blocks heat treated in the factory, were chosen for experiment. The heat treatment of rubberwood was carried out in a small-size plant equipped with a heating kiln with a maximum capacity of 0.3 m3 of wood per treatment. The treatment was started at an initial temperature of 60 °C and heated to 105 °C. And then the temperature was increased up to 185 °C or to 215 °C by increasing by 10 °C every hour and maintained over a setting period, meanwhile, stream was continuously introduced into the kiln as a shielding gas. When the heat-treatment temperature was increased to 185 °C or 215 °C, the kiln’s temperature maintained over 3 h. The condensable effluent samples were taken directly from the kiln’ flue-gas line by means of a condensable system (circulating condensate water) during the heat-treatment processing, and they were collected at the end of each 10 min heating temperature of 125 °C, 145 °C, 175 °C, 185 °C, 215 °C (Fig. 1). After each sample collection, the collected condenser was carefully washed. The selected heat-treatment temperatures (185 °C or 215 °C) in this study were consistent with the technical parameters used in heat-treatment production process of plant. The effluent samples were taken directly at the end of each 10 min heating temperature and time of 185 °C/1 h, 185 °C/3 h, 215 °C/1 h, 215 °C/3 h, and the collection method was similar with above. The wood samples used before and after heat treatment was oven dried for 12 h at 60 ℃ and 12 h at 103 ± 2 ℃ to a constant weight. The mass loss during heat treatment was calculated as follows:
where m1 was the oven dry weight of wood before treatment and m2 was the oven dry weight of wood after treatment.
Chemical analysis
The condensable effluent samples were collected and extracted using ethyl acetate at a volume ratio of 1:1, and the captured components were injected into a sample cube for further GC–MS analysis. The GC–MS analysis was carried out on a Agilent 7890B-7000B (Agilent Technologies Inc., USA) with a 30 m Hp-5 column, 0.32 mm i.d., 1.0 μm thickness, with the following temperature program: the temperature program of GC began at 50 °C and increased at the rate of 20 °C/min until 180 °C, and 5 °C/min until 280 °C (20 min) was reached, helium as the carrier gas with a flow rate of 0.8 mL/min, splitless. The program of MS was scanned over a mass range of m/z 20–500, with an ionizing voltage of 70 eV and an ionization current of 150 μA of electron ionization. The ion source temperature was 230 °C, the quadrupole temperature was 150 °C. The mass spectra were compared with the National Institute of Standards Library (2011) database to determine the pattern of organic compounds, whose matching rate is more than 90%, and the relative amounts was calculated using the area normalization method. The pH of the effluents condensated by VOCs of rubberwood from different heat-treatment stages was performed using a Mettler pH meter.
Results and discussion
Effluents condensated by VOCs during different heat-treated rubberwood stages
Some VOCs compounds emitted during the heat-treatment process of rubberwood were condensated to effluents by the cooling device and the relative amount of effluents at different heat-treatment stages were measured via gas chromatography–mass spectrometry (see Fig. 2 and Table 1).
The rubber trees were planted for the production of latex, and the rubberwood was rich in starch, soluble sugars and other carbohydrates. The hemicellulose of wood decomposed easily during heat treatment, and the acetic acid formed by deacetylation acted as a catalyst to promote the degradation of carbohydrates [22]. Hemicellulose started to degrade at relatively low temperatures, forming phenols and aldehydes due to the cleavage of acetic acid from the acetyl side chain [23, 24]. It showed that the main components of effluents released at 125 ℃ were phenol compounds and aldehyde compounds, accounting for 41.64% and 16.93% of the total, respectively (see Fig. 2). The amounts of phenols were the largest at 125 ℃, dramatically decreased to 4.75% at 145 ℃, and then gradually increased from 145 to 215 ℃. Due to with the increase of heat treated temperature, the lignin began to thermally degrade. Lignin is composed of some functional groups, such as phenolic hydroxyl groups, aliphatic hydroxyl groups and so on, and the phenols were mainly attributed to the cleavage of β-O-4 linkages in the lignin [15]. Among the phenols, the most abundant amount was (E)-2, 6-dimethoxy-4-(prop-1-en-1-yl)phenol (24.51%), which was the reactant of vanillin [25], thus the vanillin was synthesized and its amount was increased to 36.23% at 175 ℃ (see Table 1).
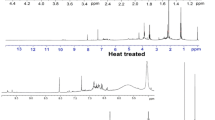
The amounts of aldehydes were emitted a much higher proportion at different heat-treatment stages of rubberwood, increasing a quantity from 125 to 145 ℃, and then declined from 175 to 215 ℃. Among the aldehydes, the most abundant amounts were vanillin and coniferyl aldehyde. The lignin was composed of three typical units, i.e., p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol [15] that possess functional groups, such as hydroxyl groups, which were easily oxidized to aldehyde. With the temperature increased, the lignin was decomposed and oxidized to form vanillin and coniferyl aldehyde. The amount of coniferyl aldehyde was most at 185 ℃, accounting for 25.68% of the total (see Table 1). The total ion chromatograms and the chemical composition of the effluent from 185 ℃ heat treatment are shown in Fig. 3 and Table 1. According to the structural comparison of the aldehyde products in the effluent with the building units of lignin, it was concluded that the aldehyde substances were mainly derived from the degradation of lignin.
With the temperature increased, the relative amounts of aldehydes decreased, while the ketones, esters and aromatics increased in Fig. 2. Some secondary reactions easily occurred under the acidic constituents of the effluents and high moisture condition. For example, the aldehydes were further oxidized to form ketones or acids at high temperature. The aldehyde and alcohol components undergo the aldol reaction to form a C–O–C bond, and hydroxyl groups combine with carbonyl groups to form hemiacetals. The reverse reaction would produce aldehydes and alcohols. The alcohol components underwent an increasing quantity from 145 to 185 ℃, and then declined to 3.13% at 215 ℃.
The amounts of acids of effluents were gradually increasing, accounting for more than 4.40% of the total at 215 ℃ (Fig. 2), which made the condensate acidic, thus the pH values ranged from 4.17 to 2.47 (Fig. 4), with the temperature increased from 125 to 215 ℃. When the temperature was at low stages, the main compounds of effluents were aliphatic acids, such as 4-hydroxy-butanoic acid. It showed that when the temperature gradually increased, the main acid was aromatic acid, and the relative amount of methylenecyclopropane carboxylic acid was decreasing from 11.41 to 2.28%, ranging from 185 to 215 ℃ (Table 1).
Comparison of effluents discharged from different technological parameters of heat treatment of rubberwood
To explore whether the effluents discharged from heat treatment of rubberwood had harmful impact on the environment, we chose the usual heat treatment technological temperature, i.e., 185 ℃ and 215 ℃ for studying, and simulating the plant production. The comparison of effluents discharged from different technological parameters of heat treatment of rubberwood is exhibited in Fig. 5.
It is shown that the effluents at 215 ℃ had more compounds than the one at 185 ℃, the reason could be that a higher heat-treatment temperature led to more intricate reactions of the wood cell wall chemicals, thus producing more complicated gas components [19]. Correspondingly, the mass loss of rubberwood was larger at 215 ℃ than the rubberwood’s at 185 ℃, accounting for 16.61% and 2.87%, respectively. As shown in Fig. 4, aldehydes had the highest proportion among the effluents at the beginning of heat treatment of rubberwood. With the heat-treatment temperature increased and the treatment time became longer, the relative amounts of aldehydes and acids declined, while the amounts of ketones, esters and phenols became much more. Because the treatment temperature became higher, the thermal-oxidation reaction among compounds was further more intense [26]. When the technological parameters of heat treatment were 215 ℃/3 h, new compounds the emerged from the effluents, such as aromatics and anhydrides, due to the severer reactions (see Table 2).
The effects of effluents on the environment and potential application
Environmental concerns related to discharges of volatile organic compounds arise since they are potential precursors for photochemical formation of ozone, other atmospheric oxidants and aerosols. As exhibited in Table 1, this study provided substantial data on the chemical composition of emissions from heat treatment of rubberwood, which simulated the production used by an industrial kiln. The aldehydes were the main compounds in the effluents at all of the different heat-treatment stages of rubberwood. Aldehydes were some of the most undesirable VOCs because of their unpleasant odor and potential toxicity [27]. Due to the acids in the VOCs generated from the heat treatment of rubberwood, the roof of the plant was corroded. According to the “China wood protection industrial development plan (2016–2020)”, the heat-treated wood anticipated to be approximately 100,000 m3 until 2020 [15]. As the main tropical plantation, the heat-treated rubberwood accounts for a certain proportion of the total production. If the effluents were discharged randomly, many of the VOCs would cause nonnegligible environmental pollution to water and soil.
Table 1 exhibits that the amounts of vanillin and coniferyl aldehyde accounted for much higher proportion in the aldehydes, and these byproducts could be used for disease treatment due to their anti-bacterial and anti-oxidant properties, which could transform waste material into things of value [28]. Since some research reported antioxidant activity of wood extractives and lignin-derived compounds [29, 30], the effluents of heat-treated rubberwood contain lots of phenols. They need to be recycled for further study on its antioxidant properties and application value and these will improve the profitability of the industrial process.
Conclusion
The characterization of effluents generated during heat-treated rubberwood can be used to undertake preliminary assessments of potential environmental impacts on cleaner heat-treated production. The results showed that the effluents emitted from heat-treated rubberwood can be classified into aldehydes, phenols, esters, ketones, acids, alcohols, alkanes, anhydride, and aromatics. The heat-treatment temperature affected the total output and chemical composition of the effluents. The amounts of aldehydes accounted for much higher proportion among all compounds at different heat-treatment stages and some of the aldehydes had application value. With the temperature increasing from 125 to 215 ℃, the amounts of acids of effluents were gradually increasing, and the pH values ranged from 4.17 to 2.47. Thus the effluents could not discharge directly, which would cause nonnegligible environmental pollution to water and soil. This study provided guidance for research of effluent disposal and transform biomass residues of effluent into things of economic value.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GC–MS:
-
Gas chromatography–mass spectrometry
- VOCs:
-
Volatile organic compounds
- MDF:
-
Medium-density fiberboard
References
Li TT, Li GJ, Lu QJ, Zhou JN, Li M, Zhang SX, Li JN (2017) Characterization of tectona grandis extractives by GC–MS and IR and their infusion into rubberwood to modify dimensional stability. BioResources 12:4500–4514. https://doi.org/10.15376/biores.12.3.4500-4514
Wang YB (2019) The planted area of rubber tree. In: Japan rubber times (ed). https://www.yunken.com/?p=65341. Accessed 9 Jan 2019
Kokutse AD, Stokes A, Bailleres H, Kokou K, Baudasse C (2006) Decay resistance of Togolese Teak (Tectona grandis) heartwood and relationship with colour. Trees 20:219–223
Jayashree S, Krishna KP, Prakash GK, Mahadevan KM (2012) Photo bleaching and dimensional stability of rubber wood esterified by fatty acid chlorides. J Wood Chem Technol 32:121–136. https://doi.org/10.1080/02773813.2011.624665
Dosdall R, Jülich WD, Schauer F (2015) Impact of heat treatment of the water reed Phragmites communis Trin. used for thatching on its stability, elasticity and resistance to fungal decomposition. Int Biodeterior Biodegrad 103:85–90. https://doi.org/10.1016/j.ibiod.2015.04.013
Gonzalez-Pena MM, Hale MDC (2010) Rapid assessment of physical properties and chemical composition of thermally modified wood by mid-infrared spectroscopy. Wood Sci Technol 45:83–102. https://doi.org/10.1007/s00226-010-0307-9
Chu DM, Xue L, Zhang Y, Kang L, Mu J (2016) Surface characteristics of poplar wood with high temperature heat treatment: wettability and surface brittleness. BioResources 11:6948–6967
Lydia SG, William JA, Sandar B, Walier D, LeBouf R, Hopke PK, Rossner A (2015) VOCs emissions from multiple wood pellet types and concentrations in indoor air. Energy Fuels 29:6485–6493. https://doi.org/10.1021/acs.energyfuels.5b01398
Günther K, Daniel S, Eugenia T, Bernhard L, Martin W, Marius CB, Alexander P (2020) Qualitative investigation on VOC-emissions from spruce (Picea abies) and larch (Larix decidua) loose bark and bark panels. Eur J Wood Wood Prod 78:403–412. https://doi.org/10.1007/s00107-020-01511-2
Liu R, Wang C, Huang AM, Lv B (2018) Characterization of odors of wood by gas chromatography–olfactometry with removal of extractives as attempt to control indoor air quality. Molecules 23:203–212. https://doi.org/10.3390/molecules23010203
Rugină C, Lupu C, Neamtiu IA, Neagu MC, Dumitraşcu I, Gurzău ES (2011) Environmental health risk assessment of VOC’s. AES Bioflux 3:99–107
Izvorni ZR (2018) Comparison of VOC emissions from natural wood and heat treated wood. Drvna Industrija 69:297–309
Wang C, Wang ZP, Qin Y, Yin XQ, Huang AM (2018) Released volatile organic compounds in southern yellow pine before and after heat treatment. Int J Environ Res Public Health 15:2579–2588. https://doi.org/10.3390/ijerph15112579
Hyttinen M, Weijo MM, Kalliokoski P (2010) Comparison of VOC emissions between air-dried and heat-treated norway spruce (Picea abies), scots pine (Pinus sylvesteris) and european aspen (Populus tremula) wood. Atmos Environ 44:5028–5033. https://doi.org/10.1016/j.atmosenv.2010.07.018
Chu DM, Zhang XY, Mu J, Avramidis S, Xue L, Li YS (2019) A greener approach to byproducts from the production of heat-treated poplar wood: analysis of volatile organic compound emissions and antimicrobial activities of its condensate. J Clean Prod 213:521–527. https://doi.org/10.1016/j.jclepro.2018.12.163
Srinivas K, Pandey KK (2012) Photodegradation of thermally modified wood. J Photochem Photobiol B 117:140–145. https://doi.org/10.1016/j.jphotobiol.2012.09.013
Severo ETD, Calonego FW, Sansígolo CA, Bond B (2016) Changes in the chemical composition and decay resistance of thermally-modified hevea brasiliensis wood. PLoS ONE 11:1353–1362. https://doi.org/10.1371/journal.pone.0151353
Granstrom K (2003) Emissions of monoterpenes and VOCs during drying of sawdust in a spouted bed. For Prod J 53:48–55. https://doi.org/10.1046/j.1439-0329.2003.00334.x
Huang WJ, Wu YF, Zhao ZJ, Yi SL, He ZB (2016) Influence of thermal treatment conditions on the release of volatile organic compounds from bamboo. BioResources 11:7296–7304
Graf N, Haas W, Bödhzelt H (2003) Characterization of gaseous emissions from a small-size industrial plant for thermal wood modification by GC/MS. In: Abstracts of the first European conference on wood modification, Ghent, Belgium, April 2003
Doddapaneni TRKC, Praveenkumar R, Tolvanen H, Palmroth MRT, Konttinen J, Rintala J (2017) Anaerobic batch conversion of pine wood torrefaction condensate. Bioresour Technol 225:299–307. https://doi.org/10.1016/j.biortech.2016.11.073
Manninena AM, Pasanenb P, Holopainena JK (2002) Comparing the VOC emissions between air-dried and heat-treated Scots pine wood. Atmos Environ 36:1763–1768
Yildiz S, Gezer ED, Yildiz UC (2006) Mechanical and chemical behavior of sprucewood modified by heat. Build Environ 41:1762–1766. https://doi.org/10.1016/s1352-2310(02)00152-8
Boonstra MJ, Acker VJ, Kegel E, Stevens M (2007) Optimisation of a two-stage heat treatment process: durability aspects. Wood Sci Technol 41:31–57. https://doi.org/10.1007/s00226-006-0087-4
Gallage NJ, Møller BL (2015) Vanillin–bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol Plant 8:40–57. https://doi.org/10.1016/j.molp.2014.11.008
Huang YX, Zhang YM, Qi Y, Yu YL, Yu WJ (2019) Identification of odorous constituents of bamboo during thermal treatment. Constr Build Mater 203:104–110. https://doi.org/10.1016/j.conbuildmat.2019.01.054
Pohleven J, Burnard MD, Kutnar A (2019) Volatile organic compounds emitted from untreated and thermally modified wood-a review. Wood Fiber Sci 51(3):231–254
Conde E, Cara C, Moure A, Ruiz E, Castro E, Domínguez H (2009) Antioxidant activity of the phenolic compounds released by hydrothermal treatments of olive tree pruning. Food Chem 114:806–812. https://doi.org/10.1016/j.foodchem.2008.10.017
Dizhbite T, Telysheva G, Jurkjane V, Viesturs U (2004) Characterization of the radical scavenging activity of lignins—natural antioxidants. Bioresour Technol 95:309–317. https://doi.org/10.1016/j.biortech.2004.02.024
Vinardell MP, Ugartondo V, Mitjans M (2008) Potential applications of antioxidant lignins from different sources. Ind Crop Prod 27:220–223. https://doi.org/10.1016/j.indcrop.2007.07.011
Acknowledgements
The authors would like to thank Cui Chen and Yuhui Ke for the preparation of the rubberwood samples used in this study.
Funding
This work was supported by the Hainan Natural Science Fund for Young Scholars (Grant number: 319QN321), and the Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (Grant number: 1630022017013).
Author information
Authors and Affiliations
Contributions
XL and JL oversaw and reviewed the overall research. TL analyzed the experimental data and wrote the manuscript while QL, GL and ML participated in all the practical activities of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Li, T., Li, G., Li, J. et al. Characterization of the effluents condensated by volatile organic compounds during heat-treated rubberwood process. J Wood Sci 66, 50 (2020). https://doi.org/10.1186/s10086-020-01897-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10086-020-01897-w