Abstract
Background
Little is known about the Canadian public’s perspective regarding clinical trials.
Methods
We surveyed 1602 Ontario and British Columbia residents to ascertain their understanding of and willingness to participate in clinical trials.
Results
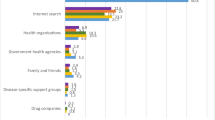
Clinical trials are regarded positively with overall perceptions that they provide societal and personal benefits. Most respondents were somewhat (49%) or very willing (19%) to participate in a clinical trial. This increased with age and level of education. It was also greater among those with poor or very poor health, those with multiple chronic conditions, and those who had previously been invited into a clinical trial, all of which were correlated with age. Still, there was room for improvement in awareness and understanding of clinical trials. Forty-three percent of those surveyed felt not very informed or not at all informed and 37% had no opinion regarding clinical trials. Respondents would most often turn to their treating physician if considering participating in a clinical trial and least often to social media.
Conclusion
While Canadians’ views about clinical trials are generally positive, they are somewhat muted and a significant minority feels poorly or not at all informed. They are less willing to participate in clinical research than Americans and are roughly equivalent to Europeans. While clinicians are the top choice for learning about clinical trials, they have little or no training and little time for this role. As we move toward integrating clinical trials into the practice setting, these issues of time, training, and resources must be addressed.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Collier R. Federal government unveils patient-oriented research strategy. CMAJ. 2011; 183: 1469.
Schroen AT, Petroni GR, Hongkun W, et al. Preliminary evaluation of factors associated with premature trial closure and feasibility of accrual benchmarks in phase III oncology trials. Clinical Trials. 2010; 7: 312–21.
Canadian Cancer Research Alliance. Report on the state of cancer clinical trials in Canada. Toronto: Canadian Cancer Research Alliance, 2011.
Anonymous. Canada’s Strategy for Patient-Oriented Research. Improving health outcomes through evidence-informed care. Ottawa: Canadian Institutes of Health Reserch, 2011.
Mansell P.Survey finds low awareness of clinical research as core NHS activity. PharmaTimes online. London, UK: PharmaTimes Media Ltd., 2012.
Massett HA, Dilts DM, Bailey R, et al. Raising Public Awareness of Clinical Trials: Development of Messages for a National Health Communication Campaign. Journal of health communication. 2017; 22: 373–85.
Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. Journal of Clinical Oncology. 2005; 23: 3112–24.
Nelson AM, Martin IG, Getz KA. Generational Value Differences Affecting Public Perceptions of and Willingness to Participate in Clinical Trials. Therapeutic Innovation & Regulatory Science. 2015; 49: 940–6.
Burns KEA, Magyarody N, Jiang D, Wald R. Attitudes and views of the general public towards research participation. Intern Med J. 2013; 43: 531–40.
Jones JM, Nyhof-Young J, Moric J, Friedman A, Wells W, Catton P. Identifying Motivations and Barriers to Patient Participation in Clinical Trials. J Cancer Educ. 2007; 21: 237–42.
Getz KA. Public perceptions are turning the corner: public’s view of clinical research has improved during the past five years, CISCRP survey reveals. Applied Clinical Trials. 2013; 22: 22.
Trauth JM, Musa D, Siminoff L, Jewell IK, Ricci E. Public Attitudes Regarding Willingness to Participate in Medical Research Studies. J Health Soc Policy. 2000; 12: 23–43.
Comis RL, Miller JD, Aldige CR, Krebs L, Stoval E. Public attitudes toward participation in cancer clinical trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003; 21: 830–5.
i-Say Ipsos Interactive Services Limited Partnership, p. https://social.i-say.com/?0
Center for Information and Study on Clinical Research Participation. 2015 Perceptions and insights study. Report on public perceptions.. Boston2015.
CISCRP - The Center for Information and Study on Clinical Research Participation. p. https://www.ciscrp.org/.
Cobb EM, Gebremariam A, Singer D, Davis MM. Public Interest in Medical Research Participation: Does It Matter if Patients or Community Members Have Helped Design the Study? Clin Transl Sci. 2015; 8: 502–5.
Mackenzie IS, Wei L, Rutherford D, et al. Promoting public awareness of randomised clinical trials using the media: the Get Randomised campaign. Br J Clin Pharmacol. 2010; 69: 128–35.
Anonymous. #WhyClinicalTrialsMatter A Social Campaign to Share the Value of Clinical Trials.
Center for Information and Study on Clinical Research Participation. 2013 Perceptions and Insights Study. Public and patient perceptions of clinical research. Report on general perceptions. Boston: Center for Information and Study on Clinical Research Participation, 2013.
Brintnall-Karabelas J, Sung S, Cadman ME, Squires C, Whorton K, Pao M. Improving Recruitment in Clinical Trials: Why Eligible Participants Decline. Journal of Empirical Research on Human Research Ethics: An International Journal. 2011; 6: 69–74.
Kelley M, James C, Alessi Kraft S, et al. Patient Perspectives on the Learning Health System: The Importance of Trust and Shared Decision Making. The American Journal of Bioethics. 2015; 15: 4–17.
Eapen ZJ, Vavalle JP, Granger CB, Harrington RA, Peterson ED, Califf RM. Rescuing clinical trials in the United States and beyond: A call for action. Am Heart J. 2013; 165: 837–47.
Rendell Jennifer M, Merritt Rowena K, Geddes J. Incentives and disincentives to participation by clinicians in randomised controlled trials. Cochrane Database of Systematic Reviews: (2007).
Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999; 52.
Califf RM.Clinical trials in crisis: Four simple methodologic fixes Commentary. Clinical Trials. 2014; 11: 626–7.
Fischer BA. A summary of important documents in the field of research ethics. Schizophr Bull. 2006; 32: 69–80.
Friesen P, Kearns L, Redman B, Caplan AL. Rethinking the Belmont Report? The American Journal of Bioethics. 2017; 17: 15–21.
Califf RM, Sugarman J. Exploring the ethical and regulatory issues in pragmatic clinical trials. Clinical Trials. 2015; 12: 436–41.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Willison, D.J., Richards, D.P., Orth, A. et al. Survey of Awareness and Perceptions of Canadians on the Benefits and Risks of Clinical Trials. Ther Innov Regul Sci 53, 669–677 (2019). https://doi.org/10.1177/2168479018805433
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479018805433