Abstract
Background
Prescription drug labeling is an authoritative source of information that guides the safe and effective use of approved medications. In many instances, however, labeling may fail to be updated as new information about drug efficacy emerges in the postmarket setting. When labeling becomes outdated, it loses its value for prescribers and undermines a core part of the FDA’s mission to communicate accurate and reliable information to patients and physicians.
Methods
We compared the number of drug uses indicated on product labels to the number of uses contained in a leading drug compendium for 43 cancer drugs approved between 1999 and 2011. We defined a “well-accepted off-label use” of a drug as one that was not approved by the FDA and received a category 1 or 2A evidence grade.
Results
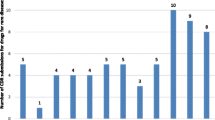
Of the 43 drugs reviewed in this study, 34 (79%) had at least one well-accepted off-label use. In total, 253 off-label uses were identified; 91% were well accepted, and 65% were in cancer types not previously represented on labeling. Off-patent drugs had more well-accepted off-label uses than brand-name drugs, on average (mean 13.7 vs 3.8, P =.018).
Conclusions
The labeling for many cancer drugs, particularly for older drugs, is outdated. Although FDA-approved labeling can never be fully aligned with real-world clinical practice, steps should be taken to better align the two when high-quality data exist. Such steps, if taken, will assist patients and prescribers in discerning which uses of drugs are supported by the highest quality evidence.
Similar content being viewed by others
References
US Department of Health and Human Services. Requirements on content and format of labeling for human prescription drug and biological products. 21 CFR §201.56. https://www.gpo.gov/fdsys/granule/CFR-2012-title21-vol4/CFR-2012-title21-vol4-sec201-56. Effective April 1, 2012. Accessed February 20, 2018.
Lindstrom J. Sources of drug information: FDA-approved labeling and other official FDA sources. Dermatol Ther. 2009;22:246–256.
Greene SM, Noah L. Debate: Off-label drug promotion and the first amendment. Univ PA Law Rev. 2015;62:239–267.
Pfister DG. Off-label use of oncology drugs: the need for more data and then some. J Clin Oncol. 2012;30:584–586.
Soares M. “Off-label” indications for oncology drug use and drug compendia: history and current status. J Oncol Pract. 2005;1:102–105.
Abernethy AP, Raman G, Balk EM, et al. Systematic review: reliability of compendia methods for off-label oncology indications. Ann Intern Med. 2009;150:336–343.
American Society of Clinical Oncology. Reimbursement for cancer treatment: coverage of off-label drug indications. J Clin Oncol. 2006;24:3206–3208.
National Comprehensive Cancer Network. About the NCCN Drugs & Biologics Compendium (NCCN Compendium®). Accessed December 5, 2017.
National Comprehensive Cancer Network. NCCN Guidelines® & Clinical Resources: development and update of the NCCN Guidelines®. https://www.nccn.org/professionals/development.aspx. Accessed March 23, 2017.
Wang B, Kesselheim AS. Characteristics of efficacy evidence supporting approval of supplemental indications for prescription drugs in United States, 2005-14: systematic review. BMJ. 2015;351:h4679.
National Comprehensive Cancer Network. NCCN Recognition Program. https://www.nccn.org/professionals/recognition.asp. Accessed May 2017.
Anthem. Clinical UM Guideline: Oncology drug treatment regimens for adults. https://www11.anthem.com/ca/medicalpolicies/guidelines/gl_pw_c166610.htm. Accessed February 2017.
Cigna. Cigna drug and biologic coverage policy: oncology medications. https://cignaforhcp.cigna.com/public/content/pdf/coveragePolicies/pharmacy/ph_1403_coveragepositioncriteria_oncology.pdf. Accessed February 2017.
United Healthcare. Oncology clinical medication coverage policy. https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Drug%20Policies/Oncology_Medication_Clinical_Coverage_Policy.pdf. Accessed February 2017.
Chen DT, Wynia MK, Moloney RM, et al. U.S. physician knowledge of the FDA-approved indications and evidence base for commonly prescribed drugs: results of a national survey. Pharmacoepidemiol Drug Saf. 2009;18:1094–1100.
Smalley W, Shatin D, Wysowski DK, et al. Contraindicated use of Cisapride: impact of food and drug administration regulatory action. JAMA. 2000;284:3036–3039.
Green AK, Wood WA, Basch EM. Time to reassess the cancer compendia for off-label drug coverage in oncology. JAMA. 2016;316:1541–1542.
Klein D, Tabarrok A. Do off-label drug practices argue against FDA efficacy requirements? A critical analysis of physicians’ argumentation for initial efficacy requirements. Am J Econ Sociol. 2008;67:743–776.
United States Food and Drug Administration. Guidance for industry: distributing scientific and medical publications on unapproved new uses—recommended practices. Rockville, MD: FDA, 2014.
Dissemination of information on unapproved/new uses for marketed drugs, biologics, and devices. Fed Regist. 1998;63:31143.
United States Food and Drug Administration. Guidance for industry: labeling for human prescription drug and biological products—implementing the PLR content and format requirements. Rockville, MD: FDA, 2013.
Prescription drug labeling improvement and enhancement initiative; request for comments and information. Fed Regist. 2013;78:8446.
US Department of Health and Human Services. Food and Drug Administration Amendments Act of 2007. Public Law 110–85. Effective September 27, 2007. https://www.gpo.gov/fdsys/pkg/PLAW-110publ85/pdf/PLAW-110publ85.pdf. Accessed February 20, 2018.
United States Food and Drug Administration. Guidance for industry: assessing user fees under the Prescription Drug User Fee Amendments of 2017. Rockville, MD: FDA, 2017.
Food and Drugs, 21 USC §355.
US Department of Health and Human Services. Adequate and well-controlled studies. 21 CFR §314.126. https://www.gpo.gov/fdsys/granule/CFR-2010-title21-vol5/CFR-2010-title21-vol5-sec314-126. Effective April 1, 2010. Accessed February 20, 2018.
United States Food and Drug Administration. Guidance for industry: responding to unsolicited requests for off-label information about prescription drugs and medical devices. Rockville, MD: FDA, 2011.
United States Food and Drug Administration. Guidance for industry: good reprint practices for the distribution of medical journal articles and medical or scientific reference publications on unapproved new uses of approved drugs and approved or cleared medical devices. Rockville, MD: FDA, 2009.
Cranston JW, Williams MA, Nielsen NH, et al.; for the Council on Scientific Affairs. Report of the Council on Scientific Affairs: unlabeled indications of Food and Drug Administration-approved drugs. Drug Inf J. 1998;32:1049–1061.
Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166:1021–1026.
Silverman E. FDA again delays rule to allow generic drug makers to change labels. Stat News. https://www.statnews.com/pharmalot/2016/05/19/fda-generic-drugs-safety/. Published May 19, 2016. Accessed December 2017.
American College of Rheumatologists. Position statement: FDA labels. https://www.rheumatology.org/Portals/0/Files/Position-Statement-FDA-Labeling.pdf. Accessed September 2017.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Shea, M.B., Stewart, M., Van Dyke, H. et al. Outdated Prescription Drug Labeling: How FDA-Approved Prescribing Information Lags Behind Real-World Clinical Practice. Ther Innov Regul Sci 52, 771–777 (2018). https://doi.org/10.1177/2168479018759662
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479018759662