Abstract
Background
Many countries incorporate direct patient reporting of adverse drug reactions (ADRs) into their pharmacovigilance systems as patients provide a different insight into drug safety compared to health care professionals. This study aimed to examine public awareness about ADR reporting in Malaysia and patients’ confidence in reporting ADRs.
Methods
Using a cross-sectional design and convenient sampling, data were collected in public areas within Kuala Lumpur, Malaysia, via face-to-face interview with a structured questionnaire. Multivariate logistic regression analysis was used to identify the significant predictors of patients’ confidence in ADR reporting.
Results
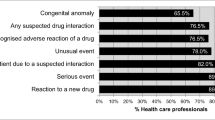
Out of 860 consented respondents achieving a response rate of 73.5%, only 69 (8%) were aware of the Malaysian ADR monitoring system. The majority (60%) of the respondents indicated they had the confidence to report ADRs. Multivariate logistic regression analysis revealed that ease in completing the ADR reporting form was the strongest variable predictive of confidence to report ADRs (odds ratio [OR], 18.45; 95% confidence interval [CI], 10.55-32.25). Increased confidence in ADR reporting was also associated with education level. Respondents with a higher education level were more likely to be confident to report ADRs compared to those with primary or no formal education (OR, 2.49; 95% CI, 0.77-8.1).
Conclusions
Lack of awareness of the ADR monitoring system is still prevalent among Malaysian patients. The ease of completing the ADR form and education level are predictive of patient confidence to report ADRs. These factors should be considered in designing public promotional activities to encourage patient contributions to pharmacovigilance.
Similar content being viewed by others
References
Blenkinsopp A, Wilkie P, Wang M, Routledge PA. Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol. 2007;63(2):148–156.
van Grootheest K, de Jong-van den Berg L. Patients’ role in reporting adverse drug reactions. Expert Opin Drug Saf. 2004;3(4):363–368.
Aziz Z, Siang TC, Badarudin NS. Reporting of adverse drug reactions: predictors of under-reporting in Malaysia. Pharmacoepidemiol Drug Saf. 2007;16(2):223–228.
Jarernsiripornkul N, Arunrot P, Krska J. Survey of patients’ experiences and their certainty of suspected adverse drug reactions. Int J Clin Pharm. 2015;37:168–174.
World Health Organization (WHO). Safety Monitoring of Medicinal Products: Reporting System for the General Public. Geneva, Switzerland: World Health Organization; 2012.
Pal SN, Olsson S, Brown EG. The Monitoring Medicines Project: a multinational pharmacovigilance and public health project. Drug Saf. 2015;38(4):319–328.
van Hunsel F, Härmark L, Pal S, Olsson S, van Grootheest K. Experiences with adverse drug reaction reporting by patients: an 11-country survey. Drug Saf. 2012;35(1):45–60.
Alshakka MA, Mohamed Izham MI, Subish P. Importance of consumer pharmacovigilance system in developing countries: a case of Malaysia. J Clin Diagn Res. 2010;4:2929–2935.
Alshakka M. Problems and challenges faced in consumer reporting of adverse drug reactions in developing countries: a case study of Yemen. Indian J Pharm Biol Res. 2014;2(3):37–43.
Elkalmi R, Hassali MA, Al-Lela OQ, Jawad Awadh AI, Al-Shami AK, Jamshed SQ. Adverse drug reactions reporting: knowledge and opinion of general public in Penang, Malaysia. J Pharm Bioallied Sci. 2013;5(3):224–228.
Fortnum H, Lee AJ, Rupnik B, Avery A; Yellow Card Study Collaboration. Survey to assess public awareness of patient reporting of adverse drug reactions in Great Britain. J Clin Pharm Ther. 2012;37(2):161–165.
Pahuja R, Shrivastava B, Sharma PK, Kishore K, Mahajan S, Sood R. Awareness on adverse drug reaction reporting system in India: a consumer survey. Am J Phytomed Clin Ther. 2014;2(12):1361–1369.
Jarernsiripornkul N, Patsuree A, Krska J. Public confidence in ADR identification and their views on ADR reporting: mixed methods study. Eur J Clin Pharmacol. 2017;73:223–231.
van Hunsel F, Passier A, van Grootheest K. Comparing patients’ and healthcare professionals’ ADR reports after media attention: the broadcast of a Dutch television programme about the benefits and risks of statins as an example. Br J Clin Pharmacol. 2009;67(5):558–564.
Alshakka MA, Ibrahim MI, Hassali MA. Do health professionals have positive perception towards consumer reporting of adverse drug reactions? J Clin Diagn Res. 2013;7(10):2181–2185.
Ting KN, Stratton-Powell DM, Anderson C. Community pharmacists’ views on adverse drug reactions reporting in Malaysia: a pilot study. Pharm World Sci. 2010;32(3):339–342.
Harmark L, Puijenbroek E, Grootheest K. Longitudinal monitoring of the safety of drugs by using a web-based system: the case of pregabalin. Pharmacoepidemiol Drug Saf. 2011;20(6):591–597.
Yamamoto M, Kubota K, Okazaki M, et al. Patients views and experiences in online reporting adverse drug reactions: findings of a national pilot study in Japan. Patient Prefer Adherence. 2015;9:173–184.
Inch J, Watson MC, Anakwe-Umeh S. Patient versus healthcare professional spontaneous adverse drug reaction reporting: a systematic review. Drug Saf. 2012;35(10):807–818.
Avery AJ, Anderson C, Bond CM, et al. Evaluation of patient reporting of adverse drug reactions to the UK “Yellow Card Scheme”: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technology Assessment. 2011;15(20):1–234.
Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101(23):1624–1632.
Herxheimer A, Crombag R, Alves TL; Direct Patient Reporting of Adverse Drug Reactions: A Fifteen-Country Survey & Literature Review. Health Action International (HAI). Available at: http://consumers.cochrane.org/sites/consumers.cochrane.org/files/public/uploads/10%20May%202010%20Report%20Direct%20Patient%20Reporting%20of%20ADRs.pdf. Accessed April 20, 2017.
McLernon DJ, Bond CM, Lee AJ, et al. Patient views and experiences of making adverse drug reaction reports to the Yellow Card Scheme in the UK. Pharmacoepidemiol Drug Saf. 2011;20(5):523–531.
McLernon DJ, Lee AJ, Hazell L, et al. Do adverse drug reaction reports differ between consumers and healthcare professionals? Drug Saf. 2009;32(10):937–938.
Leone R, Moretti U, D’Incau P, et al. Effect of pharmacist involvement on patient reporting of adverse drug reactions: first Italian study. Drug Saf. 2013;36(4):267–276.
Bandekar MS, Anwikar SR, Kshirsagar NA. Quality check of spontaneous adverse drug reaction reporting forms of different countries. Pharmacoepidemiol Drug Saf. 2010;19:1181–1185.
Harmark L, Lie-Kwie M, Berm L, et al. Patients’ motives for participating in active post-marketing surveillance. Pharmacoepidemiol Drug Saf. 2013;22(1):70–76.
McLernon DJ, Bond CM, Hannaford PC, et al. Adverse drug reaction reporting in the UK: a retrospective observational comparison of yellow card reports submitted by patients and healthcare professionals. Drug Saf. 2010;33(9):775–788.
Al Dweik R, Stacey D, Kohen D, Yaya S. Factors affecting patient reporting of adverse drug reactions: a systematic review. Br J Clin Pharmacol. 2017;83(4):875–883.
van Grootheest K, De Graaf L, de Jong-van den Berg LTW. Consumer adverse drug reaction reporting: a new step in pharmacovigilance? Drug Saf. 2003;26(4):211–217.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hariraj, V., Aziz, Z. Patient Reporting of Adverse Drug Reactions (ADRs): Survey of Public Awareness and Predictors of Confidence to Report. Ther Innov Regul Sci 52, 757–763 (2018). https://doi.org/10.1177/2168479017745025
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479017745025