Abstract
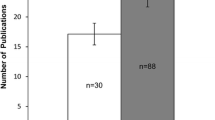
Mergers and acquisitions, the convergence of clinical research into clinical practice, more effective site selection and management practices, and efforts to improve investigator competence and credentials are all expected to contribute to consolidation in the global investigative site landscape during the coming 3 to 5 years. Tufts CSDD conducted an analysis of the FDA’s Bioresearch Monitoring Information System (BMIS) to gather baseline data with which to monitor this anticipated structural change. More than half-a-million records were analyzed on clinical investigators who have filed at least 1 form 1572 with the FDA annually between 2008 and 2015. The results show that the global landscape of unique FDA-regulated principal investigators remains highly fragmented, nascent, and unstable. Principal investigators who file the lowest volume of 1572 forms each year make up the highest proportion of the global landscape, and one-third of all principal investigators each year are first-time filers. Each year, 40% of principal investigators drop out of the clinical research enterprise. However, there are signs that the landscape is beginning to scale and mature, particularly among investigators in North America. In addition, the rate of globalization is slowing and shifting, where recent growth in the number of active principal investigators in Europe has outpaced that in North America and in the rest of world. The implications of this study and future areas of research are discussed.
Similar content being viewed by others
References
Korieth K. CROs begin consolidating site landscape. CenterWatch Monthly Newsletter. 2016;23:7–11.
Brennan Z. CROs call for better ways to leverage investigative site relationships. Outsourcing Pharma. Published February 3, 2015. www.outsourcing-pharma.com/content/view/print/1045105.
Zyla G, Redfearn S. Introducing site optimization 3.0. CenterWatch Monthly Newsletter. 2016;23:11–14.
Redfearn S. Introducing site optimization 3.0. CenterWatch Monthly Newsletter. 2016;23:11–14 Ibid.
Alsumidaie M. TransCelerate vs. ACRES site accreditation initiatives. Applied Clinical Trials Online, November 18, 2016. www.appliedclinicaltrialsonline.com/print/288579?page=full.
Korieth K. Integrated research partnerships build momentum. CenterWatch Monthly Newsletter. 2015;22:7–11.
Getz K, Zuckerman R. Today’s global site landscape. Appl Clin Trials. 2010;19:34–38.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Getz, K., Brown, C., Stergiopoulos, S. et al. Baseline Assessment of a Global Clinical Investigator Landscape Poised for Structural Change. Ther Innov Regul Sci 51, 575–581 (2017). https://doi.org/10.1177/2168479017701504
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479017701504