Abstract
Background
Adverse drug reactions (ADRs) are notoriously underreported within health care facilities. In 2009–2010, ADRs were detected in only 0.5% of patients at the authors’ institution, a pediatric hospital in the Midwestern United States. Additionally, historical ADRs were often inaccurately or incompletely documented in the medical record. An integrative Drug Safety Service (DSS) was implemented to improve the detection and accurate documentation of ADRs.
Methods
The DSS incorporated standardized ADR terminology, computerized triggers to identify ADRs, and a simplified voluntary reporting system within the facility. The DSS staff provided extensive hospital staff education on ADR reporting and the role of the DSS. The primary aim of this report was to assess the impact of the DSS on the number of ADRs reported at the authors’ institution. The secondary aims were to evaluate the mechanisms by which patients with ADRs were identified and to assess the accuracy of ADR documentation after implementation of the DSS.
Results
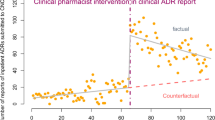
A significant increase was observed (slope, 6.01; P <.001) in ADR detection after implementation of the DSS, with a greater than 4-fold increase from 10 cases per 10,000 admissions before initiation to 41 cases per 10,000 admissions after DSS implementation. Computerized triggers, International Classification of Diseases, 9th Edition (ICD-9) codes associated with ADRs, and the DSS identified 33%, 33%, and 24% of ADRs, respectively, while voluntary reporting only detected 9% of ADRs.
Conclusions
Implementation of a multifaceted, interdisciplinary DSS was more effective in detecting ADRs than voluntary reporting alone. A proactive approach to ADR detection resulted in a significant increase in the identification and evaluation of ADRs.
Similar content being viewed by others
References
World Health Organization. Medicines: safety of medicines—adverse drug reactions. Fact sheet no. 293. October 2008. Available at: https://www.who.int/mediacentre/factsheets/fs293/en/. Accessed June 6, 2013.
Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol. 2001;52(1):77–83.
Le J, Nguyen T, Law AV, Hodding J. Adverse drug reactions among children over a 10-year period. Pediatrics. 2006;118(2):555–562.
Weiss J, Krebs S, Hoffmann C, et al. Survey of adverse drug reactions on a pediatric ward: a strategy for early and detailed detection. Pediatrics. 2002; 110 (2 Pt 1):254–257.
Pirmohamed M, Breckenridge AM, Kitterignham NR, Park BK. Adverse drug reactions. BMJ. 1998;316(7140):1295–1298.
Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68(9):1781–1790.
Kessler DA. Introducing MEDWatch: a new approach to reporting medication and device adverse effects and product problems. JAMA. 1993;269(21):2765–2768.
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245.
Belton KJ, Lewis SC, Payne S, Rawlins MD, Wood SM. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom. Br J Clin Pharmacol. 1995;39(3):223–226.
Eland IA, Belton KJ, van Grootheest AC, Meiners AP, Rawlins MD, Stricker BH. Attitudinal survey of voluntary reporting of adverse drug reactions. Br J Clin Pharmacol. 1999;48(4):623–627.
Herdeiro MT, Figueiras A, Polónia J, Gestal-Otero JJ. Physicians’ attitudes and adverse drug reaction reporting: a case-control study in Portugal. Drug Saf. 2005;28(9):825–833.
Azaz-Livshits T, Levy M, Sadan B, Shalit M, Geisslinger G, Brune K. Computerized survelliance of adverse drug reactions in hospital: pilot study. Br J Clin Pharmacol. 1998;45(3):309–314.
Evans RS, Pestotnik SL, Classen DC, Bass SB, Burke JP. Prevention of adverse drug events through computerized surveillance. Proc Annu Symp Comput Appl Med Care. 1992:437–441.
Pushkin R, Frassetto L, Tsourounis C, Segal ES, Kim S. Improving the reporting of adverse drug reactions in the hospital setting. Postgrad Med. 2010;122(6):154–164.
Yoon D, Park MY, Choi NK, Park BJ, Kim JH, Park RW. Detection of adverse drug reaction signals using an electronic health records database: comparison of the Laboratory Extreme Abnormality Ratio (CLEAR) algorithm. Clin Pharmacol Ther. 2012;91(3):467–474.
Figueiras A, Herdeiro MT, Polónia J, Gestal-Otero JJ. An educational intervention to improve physician reporting of adverse drug reactions: a cluster-randomized controlled trial. JAMA. 2006;296(9):1086–1093.
Smyth RM, Gargon E, Kirkham J, et al. Adverse drug reactions in children: a systematic review. PLoS One. 2012;7(3):e24061.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goldman, J.L., Sullins, A., Sandritter, T. et al. Pediatric Pharmacovigilance: Enhancing Adverse Drug Reaction Reporting in a Tertiary Care Children’s Hospital. Ther Innov Regul Sci 47, 566–571 (2013). https://doi.org/10.1177/2168479013499153
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479013499153