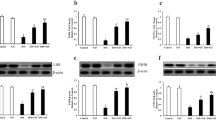
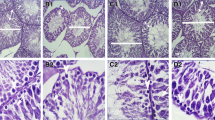
Abstract
The aim of this study was to compare the effect of synthetic estrogen (E2) with a phytoestrogen and genistein in ameliorating type 2 diabetes mellitus (T2D)-mediated testicular dysfunction in mice. The streptozotocin (STZ)-induced type 2 diabetic mice were treated exogenously with either E2 or genistein for 2 durations and compared their effects on testicular activities, serum glucose, and insulin level. Type 2 diabetic mice treated with E2 for only short term (14 days) improved regressive changes in the testicular histology by increasing testosterone synthesis and improving insulin sensitivity, whereas those treated for longer duration (28 days) failed to improve testicular dysfunctions. On the other hand, genistein treated for both short- and long term was useful in improving T2D-induced adverse effects on testicular functions. This study further suggests that treatment with genistein improves spermatogenesis in type 2 diabetic mice by increasing insulin-induced formation of lactate and antioxidative enzymes, which contributes to prevent germ cell apoptosis. Thus, genistein can be used to ameliorate T2D-induced testicular dysfunction.
Similar content being viewed by others
References
Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375(9712):408–418.
Talaei M, Pan A. Role of phytoestrogens in prevention and management of type 2 diabetes. World J Diabetes. 2015;6(2):271–283.
Ray NF, Thamer M, Taylor T, et al. Hospitalization and expenditures for the treatment of general medical conditions among the US diabetic population in 1991. J Clin Endocrinol Metabol. 1996;81(10):3671–3679.
Baccetti B, La Marca A, Piomboni P, et al Insulin-dependent diabetes in men is associated with hypothalamo-pituitary derangement and with impairment in semen quality. Hum Reprod. 2002;17(10):2673–2677.
Barros RP, Machado UF, Gustafsson JÅ. Estrogen receptors: new players in diabetes mellitus. Trends Mol Med. 2006;12(9):425–431.
Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48(1):1–9.
Yu D, Zheng W, Cai H, et al. Long-term diet quality and risk of type 2 diabetes among urban Chinese adults. Diabetes Care. 2017;41(4):723–730.
Alves MG, Martins AD, Rato L, et al. Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim Biophys Acta. 2013;1832(5):626–635.
Meccariello R, Battista N, Bradshaw HB, et al. Updates in reproduction coming from the endocannabinoid system. Int J Endocrinol. 2014;2014:412354.
Pentti K, Tuppurainen MT, Honkanen R, et al. Hormone therapy protects from diabetes: the Kuopio osteoporosis risk factor and prevention study. Eur J Endocrinol. 2009;160(6):979–983.
Kacker R, Traish AM, Morgentaler A. Estrogens in men: clinical implications for sexual function and the treatment of testosterone deficiency. J Sex Med. 2012;9(6):1681–1696.
Le May C, Chu K, Hu M, et al. Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103(24):9232–9237.
Oliveira PF, Alves MG, Rato L, et al. Influence of 5α-dihydrotestosterone and 17β-estradiol on human Sertoli cells metabolism. Int J Androl. 2011;34(6 pt 2):e612–e620.
Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy. Cardiovasc Toxicol. 2001;1(3):181–193.
Hamden K, Elfeki A, Ayadi F, et al. Therapeutic effect of phytoecdysteroids rich extract from Ajuga iva on alloxan induced diabetic rats liver, kidney and pancreas. Biofactors. 2008;33(3):165–175.
Long L, Wang J, Lu X, et al. Protective effects of scutellarin on type II diabetes mellitus-induced testicular damages related to reactive oxygen species/Bcl-2/Bax and reactive oxygen species/microcirculation/staving pathway in diabetic rat. J Diabetes Res. 2015;2015:252530.
Davis SR. Phytoestrogen therapy for menopausal symptoms?: there’s no good evidence that it’s any better than placebo. Br Med J. 2001;323(7309):354–355.
Murkies AL, Wilcox G, Davis SR. Phytoestrogens 1. J Clin Endocrinol Metabol. 1998;83(2):297–303.
Ososki AL, Kennelly EJ. Phytoestrogens: a review of the present state of research. Phytother Res. 2003;17(8):845–869.
Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76(6):1191–1201.
Behloul N, Wu G. Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol. 2013;698(1):31–38.
Shaterzadeh H, Bürger-Mendonça M, Alonso A, et al. The antioxidant effect of genistein on the in vitro metal-mediated formation of free radicals. Clin Biochem. 2011;13(44):S226.
Elmarakby AA, Ibrahim AS, Faulkner J, et al. Tyrosine kinase inhibitor, genistein, reduces renal inflammation and injury in streptozotocin-induced diabetic mice. Vascul Pharmacol. 2011;55(5-6):149–156.
Kwon S, Hess RA, Bunick D, et al. Rooster testicular germ cells and epididymal sperm contain P450 aromatase. Biol Reprod. 1995;53(6):1259–1264.
Reed MJ, Meszaros K, Entes LJ, et al. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49(11):1390–1394.
Zhang M, Lv XY, Li J, et al. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2009;2008:704045.
Arora S, Ojha SK, Vohora D. Characterisation of streptozotocin induced diabetes mellitus in swiss albino mice. Global J Pharmacol. 2009;3(2):81–84.
Skovsø S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Invest. 2014;5(4):349–358.
Guilford BL, Ryals JM, Lezi E, et al. Dorsal root ganglia mitochondrial biochemical changes in non-diabetic and streptozotocin-induced diabetic mice fed with a standard or high-fat diet. J Neurol Neurosci. 2017;8(2):pii: 180.
Bryzgalova G, Lundholm L, Portwood N, et al. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295(4):E904–E912.
Verma R, Krishna A. Effect of Letrozole, a selective aromatase inhibitor, on testicular activities in adult mice: both in vivo and in vitro study. Gen Comp Endocrinol. 2017;241:57–68.
Russell LD, Ettlin RA, Hikim APS, et al. Histopathology of testis. In: Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990:210–264.
Hess MF, Roser JF. Immunocytochemical localization of cytochrome P450 aromatase in the testis of prepubertal, pubertal, and postpubertal horses. Theriogenology. 2004;61(2-3):293–299.
Narayana K, Al-Bader M, Mousa A, et al. Molecular effects of chemotherapeutic drugs and their modulation by antioxidants in the testis. Eur J Pharmacol. 2012;674(2-3):207–216.
Kilarkaje N, Al-Bader MM. Diabetes-induced oxidative DNA damage alters p53-p21CIP1/Waf1 signaling in the rat testis. Reprod Sci. 2015;22(1):102–112.
Tousson E, Ali EM, Ibrahim W, et al. Proliferating cell nuclear antigen as a molecular biomarker for spermatogenesis in PTU-induced hypothyroidism of rats. Reprod Sci. 2011;18(7):679–686.
Anjum S, Krishna A, Tsutsui K. Inhibitory roles of the mammalian GnIH ortholog RFRP3 in testicular activities in adult mice. J Endocrinol. 2014;223(1):79–91.
Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275.
Banerjee A, Anjum S, Verma R, Krishna A. Alteration in expression of estrogen receptor isoforms alpha and beta, and aromatase in the testis and its relation with changes in nitric oxide during aging in mice. Steroids. 2012;77(6):609–620.
Van Dijk TH, Laskewitz AJ, Grefhorst A, et al. A novel approach to monitor glucose metabolism using stable isotopically labelled glucose in longitudinal studies in mice. Lab Anim. 2013;47(2):79–88.
Sarkar D, Singh SK. Neonatal hypothyroidism affects testicular glucose homeostasis through increased oxidative stress in prepubertal mice: effects on GLUT3, GLUT8 and Cx43. Andrology. 2017;5(4):749–762.
Tanishima K, Gao SX, Yamamoto R, et al. Biochemical and enzymological study of lactate dehydrogenase isoenzymes from commercial quality control sera and several animal tissue sources. Eur J Clin Chem Clin Biochem. 1995;33(11):865–868.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358.
Das K, Samanta L, Chainy GBN. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Ind J Biochem Biophys. 2000;37:201–204.
Aebi H. Catalase. In: Bergmeyer HU (Ed.) Methods of Enzymatic Analysis. 2nd ed. 1974;2:673–684.
Lee YS, Cha BY, Saito K, et al. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem Pharmacol. 2010;79(11):1674–1683.
Steger RW, Rabe MB. The effect of diabetes mellitus on endocrine and reproductive function. Proc Soc Exp Biol Med. 1997;214(1):1–10.
Kühn-Velten N, Waldenburger D, Staib W. Evaluation of steroid biosynthetic lesions in isolated leydig cells from the testes of streptozotocin-diabetic rats. Diabetologia. 1982;23(6):529–533.
Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000;97(23):12735–12740.
Kianifard D, Sadrkhanlou RA, Hasanzadeh S. The histological, histomorphometrical and histochemical changes of testicular tissue in the metformin treated and untreated streptozotocin-induced adult diabetic rats. Vet Res Forum. 2011;2(1):13–24.
Mäkinen S, Mäkelä S, Weihua Z, et al. Localization of oestrogen receptors alpha and beta in human testis. Mol Hum Reprod. 2001;7(6):497–503.
Chieffi P, D’Amato LC, Guarino F, et al. 17β-estradiol induces spermatogonial proliferation through mitogen-activated protein kinase (extracellular signal-regulated kinase 1/2) activity in the lizard (Podarcis s. sicula). Mol Reprod Dev. 2002;61(2):218–225.
Pak TR, Lynch GR, Tsai PS. Estrogen accelerates gonadal recrudescence in photo-regressed male Siberian hamsters. Endocrinol. 2002;143(10):4131–4134.
O’donnell L., Robertson KM, Jones ME, et al. Estrogen and spermatogenesis. Endocrine Rev. 2001;22(3):289–318.
Nadal A, Alonso-Magdalena P, Soriano S, et al. The pancreatic β-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304(1-2):63–68.
Adlercreutz H, Hämäläinen E, Gorbach S, Goldin B. Dietary phyto-oestrogens and the menopause in Japan. Lancet. 1992;339(8803):1233.
Shanle EK, Hawse JR, Xu W. Generation of stable reporter breast cancer cell lines for the identification of ER subtype selective ligands. Biochem Pharmacol. 2011;82(12):1940–1949.
Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1(1):15–24.
Gilbert ER, Liu D. Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic β-cell function. Food Funct. 2013;4(2):200–212.
Pereira B, Fernando L, Costa RB, et al. Hormonal regulation of superoxide dismutase, catalase, and glutathione peroxidase activities in rat macrophages. Biochem Pharmacol. 1995;50(12):2093–2098.
Rocha CS, Martins AD, Rato L, et al. Melatonin alters the glycolytic profile of Sertoli cells: implications for male fertility. Mol Hum Reprod. 2014;20(11):1067–1076.
Choubey M, Ranjan A, Bora PS, et al. Direct actions of adiponectin on changes in reproductive, metabolic, and anti-oxidative enzymes status in the testis of adult mice. Gen Comp Endocrinol. 2019;279:1–1.
Whitten PL, Naftolin F. Reproductive actions of phytoestrogens. Baillieres Clin Endocrinol Metab. 1998;12(4):667–690.
Huang R, Singh M, Dillon GH. Genistein directly inhibits native and recombinant NMDA receptors. Neuropharmacology. 2010;58(8):1246–1251.
El-Kordy EA, Alshahrani AM. Effect of genistein, a natural soy isoflavone, on pancreatic β-cells of streptozotocin-induced diabetic rats: histological and immunohistochemical study. J Microsc Ultrastruct. 2015;3(3):108–119.
Oliveira JS, Silva AAN, Silva Junior VA. Phytotherapy in reducing glycemic index and testicular oxidative stress resulting from induced diabetes: a review. Brazilian J Biol. 2017;77(1):68–78.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, R., Samanta, R. & Krishna, A. Comparative Effects of Estrogen and Phytoestrogen, Genistein on Testicular Activities of Streptozotocin-Induced Type 2 Diabetic Mice. Reprod. Sci. 26, 1294–1306 (2019). https://doi.org/10.1177/1933719118815576
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719118815576