Abstract
Introduction
Women threatening premature delivery receive synthetic glucocorticoids (sGC) to accelerate fetal lung maturation, reducing neonatal mortality and morbidity. Few investigations have explored potential long-term offspring side effects. We previously reported increased pericardial fat and liver lipids in 10-year-old (human equivalent 40 years) male baboons exposed to 3 antenatal sGC courses. We hypothesized middle-aged sGC male offspring show obesity-related morphometric changes.
Methods
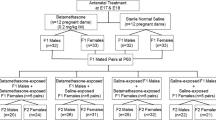
Pregnant baboons received courses of 2 betamethasone injections (175 μg·kg−1 ·d−1 intramuscular) at 0.6, 0.64, and 0.68 gestation. At 10 to 12.5 years, we measured morphometrics and serum lipids in 5 sGC-exposed males and 10 age-matched controls. We determined whether morphometric parameters predicted amount of pericardial fat or lipids. Life-course serum lipids were measured in 25 males (7-23 years) providing normal regression formulas to compare sGC baboons’ lipid biological and chronological age.
Results
Birth weights were similar. When studied, sGC-exposed males showed a steeper weight increase from 8 to 12 years and had increased waist and hip circumferences, neck and triceps skinfolds, and total and low-density lipoprotein cholesterol. Triceps skinfold correlated with apical and midventricular pericardial fat thickness, hip and waist circumferences with insulin.
Conclusions
Triceps skinfold and waist and hip circumferences are useful biomarkers for identifying individuals at risk for obesity and metabolic dysregulation following fetal sGC exposure. Prenatal sGC exposure predisposes male offspring to internal adiposity, greater body size, and increased serum lipids. Results provide further evidence for developmental programming by fetal sGC exposure and call attention to potential emergence of adverse life-course effects.
Similar content being viewed by others
References
American College of Obstetricians and Gynecologists. Committee opinion no. 677 summary: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2016;128(4):940–941.
Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454.
Audette MC, Challis JRG, Jones RL, Sibley CP, Matthews SG. Synthetic glucocorticoid reduces human placental system a transport in women treated with antenatal therapy. J Clin Endocrinol Metab. 2014;99(11):E2226–E2233.
Kuo AH, Li J, Li C, et al. Prenatal steroid administration leads to adult pericardial and hepatic steatosis in male baboons. Int J Obes. 2017;41(8):1299–1302.
Seckl JR. Glucocorticoids, developmental “programming” and the risk of affective dysfunction. Prog Brain Res. 2008;167:17–34.
Fletcher AJ, McGarrigle HH, Edwards CM, Fowden AL, Giussani DA. Effects of low dose dexamethasone treatment on basal cardiovascular and endocrine function in fetal sheep during late gestation. J Physiol. 2002;545(pt 2):649.
Luo ZC, Xiao L, Nuyt AM. Mechanisms of developmental programming of the metabolic syndrome and related disorders. World J Diabetes. 2010;1(3):89–98.
Crudo A, Petropoulos S, Suderman M, et al. Effects of antenatal synthetic glucocorticoid on glucocorticoid receptor binding, DNA methylation, and genome-wide mRNA levels in the fetal male hippocampus. Endocrinology. 2013;154(11):4170–4181.
Luo ZC, Fraser WD, Julien P, et al. Tracing the origins of “fetal origins” of adult diseases: programming by oxidative stress? Med Hypotheses. 2006;66(1):38–44.
Cianfarani S. Foetal origins of adult diseases: just a matter of stem cell number? Med Hypotheses. 2003;61(3):401–404.
Schlabritz-Loutsevitch NE, Lopez-Alvarenga JC, Comuzzie AG, et al. The prolonged effect of repeated maternal glucocorticoid exposure on the maternal and fetal leptin/insulin-like growth factor axis in Papio species. Reprod Sci. 2009;16(3):308–319.
Rodriguez JS, Zürcher NR, Keenan KE, Bartlett TQ, Nathanielsz PW, Nijland MJ. Prenatal betamethasone exposure has sex specific effects in reversal learning and attention in juvenile baboons. Am J Obstet Gynecol. 2011;204(6):545.e1–545.e10.
Koenen SV, Mecenas CA, Smith GS, Jenkins S, Nathanielsz PW. Effects of maternal betamethasone administration on fetal and maternal blood pressure and heart rate in the baboon at 0.7 of gestation. Am J Obstet Gynecol. 2002;186(4):812–817.
Blanco CL, Moreira AG, McGill-Vargas LL, Anzueto DG, Nathanielsz P, Musi N. Antenatal corticosteroids alter insulin signaling pathways in fetal baboon skeletal muscle. J Endocrinol. 2014;221(2):253–260.
Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol. 2003;547(1):117–123.
Dahlgren J, Nilsson C, Jennische E, et al. Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab. 2001;281(2):E326–E334.
Drake AJ, Raubenheimer PJ, Kerrigan D, McInnes KJ, Seckl JR, Walker BR. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology. 2010;151(4):1581–1587.
Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clinical Investigat. 1998;101(10):2174.
Cleasby ME, Livingstone DEW, Nyirenda MJ, Seckl JR, Walker BR. Is programming of glucocorticoid receptor expression by prenatal dexamethasone in the rat secondary to metabolic derangement in adulthood? Eur J Endocrinol. 2003;148(1):129–138.
Long NM, Shasa DR, Ford SP, Nathanielsz PW. Growth and insulin dynamics in two generations of female offspring of mothers receiving a single course of synthetic glucocorticoids. Am J Obstet Gynecol. 2012;207(3):203.e1–203.e8.
Berry MJ, Jaquiery AL, Oliver MH, Harding JE, Bloomfield FH. Antenatal corticosteroid exposure at term increases adult adiposity: an experimental study in sheep. Acta Obstet Gynecol Scand. 2013;92(7):862–865.
Long NM, Smith DT, Ford SP, Nathanielsz PW. Elevated glucocorticoids during ovine pregnancy increase appetite and produce glucose dysregulation and adiposity in their granddaughters in response to ad libitum feeding at 1 year of age. Am J Obstet Gynecol. 2013;209(4):353.e1–353.e9.
de Vries A, Holmes MC, Heijnis A, et al. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest. 2007;117(4):1058–1067.
Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856–1862.
Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–1727.
Williamson DF. Descriptive epidemiology of body weight and weight change in U.S. adults. Ann Intern Med. 1993;119(7 pt 2):646–649.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. 2013;(131):1–8.
Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M. The aging baboon: comparative demography in a non-human primate. PNAS. 2002;99(14):9591–9595.
Coelho AM. Baboon dimorphism: growth in weight, length and adiposity from birth to 8 years of age. In: Watts ES, ed. Nonhuman Primate Models for Human Growth and Development. New York, NY: Alan R. Liss; 1985:125–159.
Mahaney MC, Leland MM, Williams-Blangero S, Marinez YN. Cross-sectional growth standards for captive baboons: II. Organ weight by body weight. J Med Primatol. 1993;22(7–8):415–427.
Leigh SR. Growth and development of baboons. In: VandeBerg JL, Williams-Blangero S, Tardif SD, eds. The Baboon in Biomedical Research. New York, NY: Developments in Primatology: Progress and Prospects. Springer; 2009:57–88.
Saydah S, Bullard KM, Chen Y, et al. Trends in Cardiovascular Disease Risk Factors by Obesity Level in Adults in the United States, NHANES 1999–2010. Obesity (Silver Spring). 2014;22(8):1888–1895.
Thompson D, Edelsberg J, Colditz GA, Bird AP, Oster G. Lifetime health and economic consequences of obesity. Arch Intern Med. 1999;159(18):2177–2183.
Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32(1):77–97.
Durnin JV, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. 1967;21(3):681–689.
Sarría A, Moreno LA, García-Llop LA, Fleta J, Morellón MP, Bueno M. Body mass index, triceps skinfold and waist circumference in screening for adiposity in male children and adolescents. Acta Paediatr. 2001;90(4):387–392.
Nooyens ACJ, Koppes LLJ, Visscher TLS, et al. Adolescent skinfold thickness is a better predictor of high body fatness in adults than is body mass index: the Amsterdam Growth and Health Longitudinal Study. Am J Clin Nutr. 2007;85(6):1533–1539.
Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics. 2005;115(1):22–27.
Dalton M, Cameron AJ, Zimmet PZ, et al. Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med. 2003;254(6):555–563.
Taylor RW, Jones IE, Williams SM, Goulding A. Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3–19 y. Am J Clin Nutr. 2000;72(2):490–495.
Murphy VE, Zakar T, Smith R, Giles WB, Gibson PG, Clifton VL. Reduced 11beta-hydroxysteroid dehydrogenase type 2 activity is associated with decreased birth weight centile in pregnancies complicated by asthma. J Clin Endocrinol Metab. 2002;87(4):1660–1668.
Mercado AB, Wilson RC, Cheng KC, Wei JQ, New MI. Prenatal treatment and diagnosis of congenital adrenal hyperplasia owing to steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1995;80(7):2014–2020.
Kuo AH, Li C, Li J, Huber HF, Nathanielsz PW, Clarke GD. Cardiac remodelling in a baboon model of intrauterine growth restriction mimics accelerated ageing. J Physiol. 2017;595(4):1093–1110.
Kuo AH, Li C, Huber HF, Schwab M, Nathanielsz PW, Clarke GD. Maternal nutrient restriction during pregnancy and lactation leads to impaired right ventricular function in young adult baboons. J Physiol. 2017;595(13):4245–4260.
Kuo AH, Li J, Li C, Huber HF, Nathanielsz PW, Clarke GD. Poor perinatal growth impairs baboon aortic windkessel function. J Dev Orig Health Dis. 2018;9(2):137–142.
Dunn E, Kapoor A, Leen J, Matthews SG. Prenatal synthetic glucocorticoid exposure alters hypothalamic-pituitary-adrenal regulation and pregnancy outcomes in mature female guinea pigs. J Physiol. 2010;588(pt 5):887–899.
Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572(pt 1):31–44.
Zambrano E, Nathanielsz PW. Mechanisms by which maternal obesity programs offspring for obesity: evidence from animal studies. Nutr Rev. 2013;71(suppl 1):S42–S54.
Plavcan JM. Sexual dimorphism in primate evolution. Am J Phys Anthropol. 2001;116(S33):25–53.
Higham JP, MacLarnon AM, Ross C, Heistermann M, Semple S. Baboon sexual swellings: information content of size and color. Horm Behav. 2008;53(3):452–462.
Asztalos EV, Murphy KE, Willan AR, et al. Multiple courses of antenatal corticosteroids for preterm birth study: outcomes in children at 5 years of age (MACS-5). JAMA Pediatr. 2013;167(12):1102–1110.
Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311–1320.
Wapner RJ. Antenatal corticosteroids for periviable birth. Semin Perinatol. 2013;37(6):410–413.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ Note
This work was performed at the institution Southwest National Primate Research Center and Texas Biomedical Research Institute, San Antonio, TX. Animal welfare legal compliance: United States Animal Welfare Act.
Rights and permissions
About this article
Cite this article
Huber, H.F., Kuo, A.H., Li, C. et al. Antenatal Synthetic Glucocorticoid Exposure at Human Therapeutic Equivalent Doses Predisposes Middle-Age Male Offspring Baboons to an Obese Phenotype That Emerges With Aging. Reprod. Sci. 26, 591–599 (2019). https://doi.org/10.1177/1933719118778794
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719118778794