Abstract
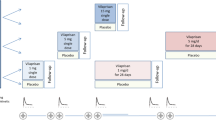
BAY 1158061 is a potent monoclonal prolactin (PRL) receptor antibody, blocking PRL receptor (PRLR)-mediated signaling in a noncompetitive manner, which was tested in a randomized, placebo-controlled multiple dose study in postmenopausal women. The objective was to investigate safety, tolerability, pharmacokinetic characteristics, and effects of BAY 1158061 on serum PRL level. The study consisted of 4 parallel groups receiving up to 3 subcutaneous (sc) administrations of BAY 1158061 or placebo in 2 different dosing regimens. Twenty-nine healthy postmenopausal women were randomized and treated with BAY 1158061 or placebo: 30 mg at 14-day interval (7 participants), 60 mg at 28-day interval (8 participants), 90 mg at 14-day interval (7 participants), and placebo (7 participants). To keep the blinding, all randomized participants received sc injections biweekly (14-day interval) on 3 occasions in the lower abdomen. The PRLR antibody showed a favorable safety and tolerability profile in postmenopausal women with no distinct differences in occurrence of adverse events in BAY 1158061 or placebo-treated participants. BAY 1158061 displayed low immunogenicity with low titers of antidrug antibodies and absence of neutralizing antidrug antibodies. Pharmacokinetics were characterized by slow absorption after sc administration with median peak plasma concentrations 7 to 11 days after first dose and about 2-fold accumulation after repeated dosing every 2 weeks. The apparent mean elimination half-life was 9 to 16 days. The PRL concentration—time profiles over 24 hours showed no differences between verum- and placebo-treated participants. Based on the data obtained, BAY 1158061 is considered a good candidate for further development in endometriosis or other PRL-mediated disease conditions.
Similar content being viewed by others
References
Marano RJ, Ben-Jonathan N. Minireview: extrapituitary prolactin: an update on the distribution, regulation and functions. Mol Endocrinol. 2014;28(5):622–633.
Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function and regulation of secretion. Physiol Rev. 2000;80(4):1523–1631.
Bellis AD, Bizzarro A, Antonio Bellastella A. Role of prolactin in autoimmune diseases. In: Sara E. Walker, Luis J. Jara, eds. Handbook of Systemic Autoimmune Diseases Vol 9; 2008:29–43. Amsterdam, Netherlands: Elsevier.
Shelly S, Boaz M, Orbach H. Prolactin and autoimmunity. Autoimmun Rev. 2012;11(6–7):A465–A470.
Bernard V, Young J, Chanson P, Binart N. New insights in prolactin: pathological implications. Nat Rev Endocrinol. 2015;11(5):265–275.
Ignacak A, Kasztelnik M, Sliwa T, Korbut RA, Rajda K, Guzik TJ. Prolactin — not only lactotropin — a “new” view of the “old” hormone. J Physiol Pharmacol. 2012;63(5):435–443.
Bernichtein S, Touraine P, Goffin V: New concepts in prolactin biology. J Endocrinol. 2010;206(1):1–11.
Sumiyoshi T. Possible dose-side effect relationship of antipsychotic drugs: relevance to cognitive function in schizophrenia. Expert Rev Clin Pharmacol. 2008;1(6):791–802.
Fitzgerald P, Dinan TG. Prolactin and dopamine: what is the connection? a review article. J Psychopharmacol. 2008;22(2 suppl):12–19.
Gerlo S, Davis JR, Mager DL, Kooijman R. Prolactin in man: a tale of two promoters. Bioassays. 2006;28(10):1051–1055.
Gourdou I, Gabou L, Paly J, Kermabon AY, Belair L, Djiane J. Development of a constitutively active mutant form of the prolactin receptor, a member of the cytokine receptor family. Mol Endocrinol. 1996;10(1):45–56.
Lee RC, Walters JA, Reyland ME, Anderson SM. Constitutive activation of the prolactin receptor results in the induction of growth factor-independent proliferation and constitutive activation of signaling molecules. J Biol Chem. 1999;274(15):10024–10034.
Goffin V, Touraine P. The prolactin receptor as a therapeutic target in human diseases: browsing new potential indications. Expert Opin Ther Targets 2015;19(9):1229–1244.
Gregoriou G, Bakas P, Vitoratos N, et al. G. Evaluation of serum prolactin levels in patients with endometriosis and infertility. Gynecol Obstet Invest. 1999;48(1):48–51.
Lima AP, Moura MD, Rosa e Silva AA. Prolactin and cortisol levels in women with endometriosis. Braz J Med Biol Res. 2006;39(8):1121–1127.
Gómez R, Abad A, Delgado F, Tamarit S, Simón C, Pellicer A. Effects of hyperprolactinemia treatment with the dopamine agonist quinagolide on endometriotic lesions in patients with endometriosis-associated hyperprolactinemia. Fertil Steril. 2011;95(3):882–888.
Craven AJ, Nixon AJ, Ashby MG, et al. Prolactin delays hair regrowth in mice. J Endocrinol. 2006;191(2):415–425.
Foitzik K, Langan EA, Paus R. Prolactin and the Skin: a Dermatological Perspective on an Ancient Pleiotropic Peptide Hormone. J Invest Dermatol. 2009;129(5):1071–1087.
Agarwal N, Machiels JP, Suarez C, et al. Phase I study of the prolactin receptor antagonist LFA102 in metastatic breast and castration-resistant prostate cancer. Oncologist. 2016;21(5):535–536.
Otto C, Särnefält A, Ljungars A, et al. A neutralizing prolactin receptor antibody whose in vivo application mimics the phenotype of female prolactin receptor-deficient mice. Endocrinology. 2015;156(11):4365–4373.
Salfeld JG. Isotype selection in antibody engineering. Nat Biotechnol. 2007;25(12):1369–1372.
Damiano JS, Rendahl KG, Karim C, et al. Neutralization of prolactin receptor function by monoclonal antibody lfa102, a novel potential therapeutic for the treatment of breast cancer. Mol Cancer Ther. 2013;12(3):295–305.
EMEA/CHMP/EWP: Guideline on Immunogenicity assessment of biotechnology-derived therapeutic proteins. 2015. Web site. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/10/WC500194507.pdf
FDA CDER-Guidance for Industry: Bioanalytical Method Validation, 2001. Web site. https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf
EMEA/CHMP/EWP: Guideline on bioanalytical method validation, 2012. Web site. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf
Shankar G, Pendley C, Stein KE. A risk-based bioanalytical strategy for the assessment of antibody immune response against biological drugs. Nat Biotechnol. 2007;25(5):555–561.
Richter WF, Jacobsen B. Subcutaneous absorption of biotherapeutics: knowns and unknowns. Drug Metab Dispos. 2014;42(11):1881–1889.
Mager EM. Target-mediated drug disposition and dynamics. Biochem Pharmacol. 2006,72(1):1–10.
Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493–507.
Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M. Pharmacokinetics, pharmacodynamics and physically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet. 2013;52(2):83–124.
Deng R, Jin F, Prabhu S, Lyer S. Monoclonal antibodies: what are the pharmacokinetic and pharmacodynamics considerations for drug development. Expert Opin Drug Metab Toxico. 2012;8(2):141–160.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ Note
Rüdiger Nave and Stefan Jodl contributed equally to this publication.
Rights and permissions
About this article
Cite this article
Nave, R., Jodl, S., Hoffmann, A. et al. Monoclonal Antibody Against Prolactin Receptor: A Randomized Placebo-Controlled Study Evaluating Safety, Tolerability, and Pharmacokinetics of Repeated Subcutaneous Administrations in Postmenopausal Women. Reprod. Sci. 26, 523–531 (2019). https://doi.org/10.1177/1933719118776806
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719118776806