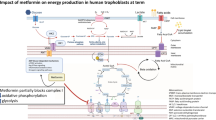
Abstract
Objective
Adenosine monophosphate—activated protein kinase (AMPK) is a cellular energy sensor whose phosphorylation increases energy production. We sought to evaluate the placenta-specific effect of AMPK activation on the handling of nutrients required for fetal development.
Methods
Explants were isolated from term placenta of 29 women (pregravid body mass index: 29.1 ± 9.9 kg/m2) and incubated for 24 hours with 0 to 100 µmol/L resveratrol or 0 to 1 mmol/L of 5-aminoimidazole-4-carboxyamide ribonucleoside (AICAR). Following treatment, uptake and metabolism of radiolabeled fatty acids and glucose were measured. Phosphorylation of AMPK was measured by Western blotting. Adenosine diphosphate (ATP) production was assessed using the mitochondrial ToxGlo assay kit. P <.05 was considered statistically significant.
Results
Resveratrol and AICAR increased AMPK phosphorylation in human placental explants. Exposure to resveratrol decreased the uptake of polyunsaturated fatty acids, arachidonic acid, and docosahexaenoic acid at 100 µmol/L (P <.0001). Fatty acid oxidation was decreased by 100 µmol/L (P <.05) resveratrol, while esterification was unchanged. Resveratrol decreased glucose uptake at the 50 and 100 µmol/L doses (P <.05). Glycolysis was not significantly affected. AICAR had similar effects, decreasing fatty acid uptake and glycolysis (P <.05). Production of ATP declined at doses found to decrease nutrient metabolism (P <.05).
Conclusions
Activation of AMPK in the human placenta leads to global downregulation of metabolism, with mitotoxicity induced at the doses of resveratrol and AICAR used to activate AMPK. Although activation of this pathway has positive metabolic effects on other tissues, in the placenta there is potential for harm, as inadequate placental delivery of critical nutrients may compromise fetal development.
Similar content being viewed by others
References
Calabuig-Navarro V, Haghiac M, Minium J, et al. Effect of maternal obesity on placental lipid metabolism. Endocrinology. 2017;158(8):2543–2555.
Visiedo F, Bugatto F, Quintero-Prado R, Cozar-Castellano I, Bartha JL, Perdomo G. Glucose and fatty acid metabolism in placental explants from pregnancies complicated with gestational diabetes mellitus. Reprod Sci. 2015;22(7):798–801.
Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG. The influence of placental metabolism on fatty acid transfer to the fetus. J Lipid Res. 2017;58(2):443–454.
Jimenez-Gomez Y, Mattison JA, Pearson KJ, et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013;18(4):533–545.
Um JH, Park SJ, Kang H, et al. AMP-activated protein kinase—deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59(3):554–563.
Saben J, Lindsey F, Zhong Y, et al. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35(3):171–177.
Chen KH, Cheng ML, Jing YH, Chiu DT, Shiao MS, Chen JK. Resveratrol ameliorates metabolic disorders and muscle wasting in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2011;301(5):E853–E863.
Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9(5):407–416.
Kulkarni SS, Canto C. The molecular targets of resveratrol. Biochim Biophys Acta. 2015;1852(6):1114–1123.
Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104(17):7217–7222.
de Ligt M, Timmers S, Schrauwen P. Resveratrol and obesity: can resveratrol relieve metabolic disturbances? Biochim Biophys Acta. 2015;1852(6):1137–1144.
Dolinsky VW, Chakrabarti S, Pereira TJ, et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim Biophys Acta. 2013;1832(10):1723–1733.
Price NL, Gomes AP, Ling AJY, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675–690.
Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77(6):1053–1063.
Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol (resVida™) supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5): doi:https://doi.org/10.1016/j.cmet.2011.1010.1002.
Roberts VH, Pound LD, Thorn SR, et al. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 2014;28(6):2466–2477.
O’Tierney-Ginn P, Roberts V, Gillingham M, et al. Influence of high fat diet and resveratrol supplementation on placental fatty acid uptake in the Japanese macaque. Placenta. 2015;36(8):903–910.
Bourque SL, Dolinsky VW, Dyck JR, Davidge ST. Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta. 2012;33(5):449–452.
Singh CK, Ndiaye MA, Ahmad N. Resveratrol and cancer: challenges for clinical translation. Biochim Biophys Acta. 2015;1852(6):1178–1185.
Mullane K, Bullough D, Shapiro D. From academic vision to clinical reality: a case study of acadesine. Trends Cardiovasc Med. 1993;3(6):227–234.
Liong S, Lappas M. Activation of AMPK improves inflammation and insulin resistance in adipose tissue and skeletal muscle from pregnant women. J Physiol Biochem. 2015;71(4):703–717.
Lim R, Barker G, Lappas M. Activation of AMPK in human fetal membranes alleviates infection-induced expression of pro-inflammatory and pro-labour mediators. Placenta. 2015;36(4):454–462.
Brass E, Hanson E, O’Tierney-Ginn PF. Placental oleic acid uptake is lower in male offspring of obese women. Placenta. 2013;34(6):503–509.
Calabuig-Navarro V, Puchowicz M, Glazebrook P, et al. Effect of omega-3 supplementation on placental lipid metabolism in overweight and obese women. Am J Clin Nutr. 2016;103(4):1064–1072.
Visiedo F, Bugatto F, Sanchez V, Cozar-Castellano I, Bartha JL, Perdomo G. High glucose levels reduce fatty acid oxidation and increase triglyceride accumulation in human placenta. Am J Physiol Endocrinol Metab. 2013;305(2):E205–E212.
Poulsen MM, Vestergaard PF, Clasen BF, et al. High-dose resveratrol supplementation in obese men. An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62(4):1186–1195.
Park EJ, Pezzuto JM. The pharmacology of resveratrol in animals and humans. Biochim Biophys Acta. 2015;1852(6):1071–1113.
Gaidhu MP, Fediuc S, Ceddia RB. 5-Aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside-induced AMP-activated protein kinase phosphorylation inhibits basal and insulin-stimulated glucose uptake, lipid synthesis, and fatty acid oxidation in isolated rat adipocytes. J Biol Chem. 2006;281(36):25956–25964.
Edwards JA, Beck M, Riegger C, Bausch J. Safety of resveratrol with examples for high purity, trans-resveratrol, resVida™. Anna N Y Acad Sci. 2011;1215(1):131–137.
Patel KR, Brown VA, Jones DJ, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70(19):7392–7399.
Poulsen MM, Fjeldborg K, Ornstrup MJ, Kjær TN, Nøhr MK, Pedersen SB. Resveratrol and inflammation: challenges in translating pre-clinical findings to improved patient outcomes. Biochim Biophys Acta. 2015;1852(6):1124–1136.
Bastianetto S, Ménard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta. 2015;1852(6):1195–1201.
Pasinetti GM, Wang J, Ho L, Zhao W, Dubner L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim Biophys Acta. 2015;1852(6):1202–1208.
Zordoky BN, Robertson IM, Dyck JR. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim Biophys Acta. 2015;1852(6):1155–1177.
Wang B, Sun J, Li L, Zheng J, Shi Y, Le G. Regulatory effects of resveratrol on glucose metabolism and T-lymphocyte subsets in the development of high-fat diet-induced obesity in C57BL/6 mice. Food Funct. 2014;5(7):1452–1463.
Cudmore MJ, Ramma W, Cai M, et al. Resveratrol inhibits the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta. Am J Obstet Gynecol. 2012;206(3):253 e210–253 e255.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Landau, D., Haghiac, M., Minium, J. et al. Activation of AMPK in Human Placental Explants Impairs Mitochondrial Function and Cellular Metabolism. Reprod. Sci. 26, 487–495 (2019). https://doi.org/10.1177/1933719118776803
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719118776803