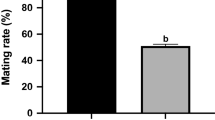
Abstract
In Western society, couples increasingly delay parenthood until later in life. Overall, studies have focused on the reproductive performance of older parents or the impact of advanced maternal age on pregnancy outcomes, but few studies have examined how advanced paternal age (APA) affects offspring health. The aim of this study was to investigate the impact of increasing paternal age on offspring reproductive performance and long-term metabolic health in a mouse model. Here, the same adult B6D2FI/J male mice were mated at 4, 12, and 18 months of age with 6-to 10-week-old naturally cycling CFI females to generate 3 offspring cohorts conceived at increasing paternal ages PA4, PA 12, and PA 18. The offspring resulting from mating the same fathers at different ages (n = 20 per age; 10 males and 10 females) were maintained up to 20 weeks of age and morphometric parameters, growth curve, and glucose tolerance were measured. We found that increasing paternal age was associated with a trend toward longer time to conception. Litter sizes were not significantly different. Reassuringly, metabolic parameters and growth curve were not different in the 3 cohorts of offspring. Most importantly, increased paternal age (PA4 vs PAI8) was associated with a statistically significant decrease in sperm concentration, sperm motility, and anogenital distance in offspring. These changes raise concerns about the potential impact of APA on the reproductive fitness in males of the next generation.
Similar content being viewed by others
References
Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol. 2004;104(4):727–733.
Luke B, Brown MB, Grainger DA, Stern JE, Klein N, Cedars MI; SART Writing Group. The effect of early fetal losses on singleton assisted-conception pregnancy outcomes. Fertil Steril. 2009; 91(6):2578–2585.
Mahmoud AM, Goemaere S, El-Garem Y, Van Pottelbergh I, Comhaire FH, Kaufman JM. Testicular volume in relation to hormonal indices of gonadal function in community-dwelling elderly men. J Clin Endocrinol Metab. 2003;88(1):179–184.
Zenzmaier C, Untergasser G, Berger P. Aging of the prostate epithelial stem/progenitor cell. Exp Gerontol, 2008;43(11):981–985.
Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33.
Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000;163(2):460–463.
Kovac JR, Addai J, Smith RP, Coward RM, Lamb DJ, Lipshultz L. The effects of advanced paternal age on fertility. Asian J Androl. 2013;15(6):723–728.
D’Onofrio BM, Rickert ME, Frans E, et al. Paternal age at child-bearing and offspring psychiatric and academic morbidity. JAMA Psychiatry, 2014;71(4):432–438.
Tsuchiya KJ, Matsumoto K, Miyachi T, et al. Paternal age at birth and high-functioning autistic-spectrum disorder in offspring. Br J Psychiatry. 2008;193(4):316–321.
Sandin S, Schendel D, Magnusson P, et al. Autism risk associated with parental age and with increasing difference in age between the parents. Mol Psychiatry. 2016;21(5):693–700.
Garrido N, Garcia-Herrero S, Meseguer M. Assessment of sperm using mRNA microarray technology. Fertil Steril. 2013;99(4):1008–1022.
Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488(7412):471–475.
Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200.
Savage T, Derraik JG, Miles HL, Mouat F, Hofman PL, Cutfield WS. Increasing paternal age at childbirth is associated with taller stature and less favourable lipid profiles in their children. Clin Endocrinol (Oxf). 2014;80(2):253–260.
Garcia-Palomares S, Navarro S, Pertusa JF, et al. Delayed fatherhood in mice decreases reproductive fitness and longevity of offspring. BiolReprod. 2009;80(2):343–349.
Garcia-Palomares S, Pertusa JF, Minarro J, et al. Long-term effects of delayed fatherhood in mice on postnatal development and behavioral traits of offspring. Biol Reprod. 2009;80(2):337–342.
Serre V, Robaire B. Paternal age affects fertility and progeny outcome in the Brown Norway rat. Fertil Steril. 1998;70(4):625–631.
Katz-Jaffe MG, Parks J, McCallie B, Schoolcraft WB. Aging sperm negatively impacts in vivo and in vitro reproduction: a longitudinal murine study. Fertil Steril. 2013;100(1):262–268. e1–e2.
Barker DJ, Mothers, Babies And Health In Later Life. 2nd ed. Glasgow, Scotland: Churchill Livingstone; 1998.
Agnish ND, Keller KA. The rationale for culling of rodent litters. Fundam Appl Toxicol. 1997;38(1):2–6.
Donjacour A, Liu X, Lin W, Simbulan R, Rinaudo PF. In vitro fertilization affects growth and glucose metabolism in a sex-specific manner in an outbred mouse model. Biol Reprod. 2014; 90(4):80.
Feuer SK, Liu X, Donjacour A, et al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology. 2014;155(5):1956–1969.
Caballero-Campo P, Buffone MG, Benencia F, et al. A role for the chemokine receptor CCR6 in mammalian sperm motility and chemotaxis. J Cell Physiol. 2014;229(1):68–78.
Travis AJ, Foster JA, Rosenbaum NA, et al. Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and fibrous sheath of murine spermatozoa. Mol Biol Cell. 1998;9(2):263–276.
Bloise E, Lin W, Liu X, etal. Impaired placental nutrient transport in mice generated by in vitro fertilization. Endocrinology. 2012;153(7):3457–3467.
Estomba H, Munoa-Hoyos I, Gianzo M, et al. Expression and localization of opioid receptors in male germ cells and the implication for mouse spermatogenesis. PLoS One. 2016; 11(3): e0152162.
Maheshwari A, Porter M, Shetty A, Bhattacharya S. Women’s awareness and perceptions of delay in childbearing. Fertil Steril. 2008;90(4):1036–1042.
Rinaudo P, Wang E. Fetal programming and metabolic syndrome. Annu Rev Physiol. 2012;74:107–130.
Dewailly D, Robert Y, Helin I, et al. Ovarian stromal hypertrophy in hyperandrogenic women. Clin Endocrinol (Oxf). 1994;41(5):557–562.
Eisenberg ML, Hsieh MH, Walters RC, Krasnow R, Lipshultz LI. The relationship between anogenital distance, fatherhood, and fertility in adult men. PLoS One. 2011;6(5):e18973.
van den Driesche S, Scott HM, MacLeod DJ, Fisken M, Walker M, Sharpe RM. Relative importance of prenatal and postnatal androgen action in determining growth of the penis and anogenital distance in the rat before, during and after puberty. Int J Androl. 2011;34(6 pt 2):e578–e586.
Moody S, Goh H, Bielanowicz A, Rippon P, Loveland KL, Itman C. Prepubertal mouse testis growth and maturation and androgen production are acutely sensitive to di-n-butyl phthalate. Endocrinology. 2013;154(9):3460–3475.
Mitchell RT, Mungall W, McKinnell C, et al. Anogenital distance plasticity in adulthood: implications for its use as a biomarker of fetal androgen action. Endocrinology. 2015;156(1):24–31.
Zhu JL, Madsen KM, Vestergaard M, Basso O, Olsen J. Paternal age and preterm birth. Epidemiology. 2005;16(2):259–262.
Astolfi P, De Pasquale A, Zonta L. Late childbearing and its impact on adverse pregnancy outcome: stillbirth, preterm delivery and low birth weight. Rev Epidemiol Sante Publique. 2005; 53(spec no 2):2S97–2S105.
Dain L, Auslander R, Dirnfeld M. The effect of paternal age on assisted reproduction outcome. Fertil Steril. 2011;95(1):1–8.
Bellver J, Garrido N, Remohi J, Pellicer A, Meseguer M. Influence of paternal age on assisted reproduction outcome. Reprod Biomed Online. 2008;17(5):595–604.
Frattarelli JL, Miller KA, Miller BT, Elkind-Hirsch K, Scott RT Jr. Male age negatively impacts embryo development and reproductive outcome in donor oocyte assisted reproductive technology cycles. Fertil Steril. 2008;90(1):97–103.
Klonoff-Cohen HS, Natarajan L. The effect of advancing paternal age on pregnancy and live birth rates in couples undergoing in vitro fertilization or gamete intrafallopian transfer. Am J Obstet Gynecol. 2004;191(2):507–514.
Robertshaw I, Khoury J, Abdallah ME, Warikoo P, Hofmann GE. The effect of paternal age on outcome in assisted reproductive technology using the ovum donation model. Reprod Sci. 2014; 21(5):590–593.
Luna M, Finkler E, Barritt J, et al. Paternal age and assisted reproductive technology outcome in ovum recipients. Fertil Steril. 2009;92(5):1772–1775.
Gunes S, Hekim GN, Arslan MA, Asci R. Effects of aging on the male reproductive system. J Assist Reprod Genet. 2016;33(4):441–454.
Jodar M, Oriola J, Mestre G, et al. Polymorphisms, haplotypes and mutations in the protamine 1 and 2 genes. Int J Androl. 2011;34(5 pt 1):470–485.
Hagaman JR, Moyer JS, Bachman ES, et al. Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci U S A. 1998;95(5):2552–2557.
Nayernia K, Adham IM, Burkhardt-Göttges E, et al. Asthenozoos-permia in mice with targeted deletion of the sperm mitochondrion-associated cysteine-rich protein (Smcp) gene. Mol Cell Biol. 2002;22(9):3046–3052.
Kim ST, Moley KH. Paternal effect on embryo quality in diabetic mice is related to poor sperm quality and associated with decreased glucose transporter expression. Reproduction. 2008; 136(3):313–322.
Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80(6):1420–1430.
Yuen RK, Merico D, Cao H, et al. Genome-wide characteristics of de novo mutations in autism. NPJ Genom Med. 2016;1:160271–1602710.
Ozkosem B, Feinstein SI, Fisher AB, O’Flaherty C. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol. 2015;5:15–23.
Shea JM, Serra RW, Carone BR, et al. Genetic and epigenetic variation, but not diet, shape the sperm methylome. Dev Cell. 2015;35(6):750–758.
Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31(33):11748–11755.
Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci USA. 2015;112(44):13699–13704.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caballero-Campo, P., Lin, W., Simbulan, R. et al. Advanced Paternal Age Affects Sperm Count and Anogenital Distance in Mouse Offspring. Reprod. Sci. 25, 515–522 (2018). https://doi.org/10.1177/1933719118759441
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719118759441