Abstract
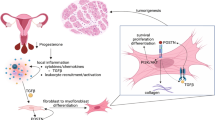
In a prior randomized controlled study, patients treated with ulipristal acetate (UPA) or placebo for 3 months had a decrease in leiomyoma size. A total of 10 patients’ tissue samples (5 placebo and 5 treated with 10 mg/d UPA) that underwent hysterectomy and tissue preservation were identified from this study. Quantitative real-time reverse transcriptase polymerase chain reaction and Western blotting were used to assess fold gene and protein expression of extracellular membrane (ECM) proteins: collagen IA (COLI A), fibronectin (FNI), and versican (VCAN) of the samples. Confirmatory immunohistochemical analysis was performed. Changes in total matrix collagen were examined using Masson trichrome staining. Multiplex measurement of the matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases was performed. Compared to placebo-treated surgical specimens, 80% of the treated specimens showed decrease in VCAN protein, 60% showed decrease in FNI, but no consistent alteration in COLI A. This effect was also supported by immunohistochemistry where leiomyoma surgical specimens demonstrated decreased amount of FNI and VCAN on UPA treatment. Increased MMP2 and decreased MMP9 in treated patient leiomyomas indicate both degradation of the matrix and inhibition of the pathway involved in matrix production. Treatment with UPA decreased fibroid volume in placebo-controlled, randomized trials. Treatment with UPA decreased gene expression and protein production in leiomyoma tissue, suggesting both an impact on water content and ECM protein concentration as a mechanism of ulipristal-mediated decrease in leiomyoma size.
Similar content being viewed by others
References
Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obset Gynecol. 2003;188(1):100–107.
Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59(1):30–52.
Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308(5728):1589–1592.
Payson M, Leppert P, Segars J. Epidemiology of myomas. Obset Gynecol Clin N Am. 2006;33(1):1–11.
Myers ER, Barber MD, Gustilo-Ashby T, Couchman G, Matchar DB, McCrory DC. Management of uterine leiomyomata: what do we really know? Obstet Gynecol. 2002;100(1):8–17.
Levens ED, Potlog-Nahari C, Armstrong AY, et al. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1129–1136.
Nieman LK, Blocker W, Nansel T, et al. Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase lib study. Fertil Steril. 2011;95(2):767–772.
Nieman LK. Treatment of uterine fibroids with the selective progesterone receptor modulator CDB-2014. https://clinicaltrials.gov/ct2/show/NCT00290251.
Donnez J, Tatarchuk TF, Bouchard P, et al; PEARL I Study Group. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366(5):409–420.
Donnez J, Tomaszewski J, Vazquez F, et al; PEARL II Study Group. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N EnglJ Med. 2012;366(5):421–432.
Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103(2):519–527.
Malik M, Britten J, Cox J, Patel A, Catherino WH. Gonadotropin-releasing hormone analogues inhibit leiomyoma extracellular matrix despite presence of gonadal hormones. Fertil Steril. 2016;105(1):214–224.
Patel A, Malik M, Britten J, Cox J, Catherino WH. Mifepristone inhibits extracellular matrix formation in uterine leiomyoma. Fer-til Steril. 2016;105(4):1102–1110.
Rosato E, Farris M, Bastianelli C. Mechanism of action of ulipristal acetate for emergency contraception: a systematic review. Front Pharmacol. 2016;6:315.
Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obset Gynecol. 2006;195(2):415–420.
Malik M, Norian J, McCarthy-Keith D, Britten J, Catherino WH. Why leiomyomas are called fibroids: the central role of extracellular matrix in symptomatic women. Sent Reprod Med. 2010;28(3):169–179.
Chegini N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med. 2010;28(3):180–203.
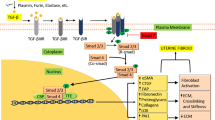
Norian JM, Malik M, Parker CY, et al. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci. 2009;16(12):1153–1164.
Fujisawa C, Castellot J Jr. Matrix production and remodeling as therapeutic targets for uterine leiomyoma. J Cell Commun Signal. 2014;8(3):179–194.
Xu Q, Ohara N, Liu J, et al. Progesterone receptor modulator CDB-2914 induces extracellular matrix metalloproteinase inducer in cultured human uterine leiomyoma cells. Mol Hum Reprod. 2008;14(3):181–191.
Xu Q, Ohara N, Chen W, et al. Progesterone receptor modulator CDB-2914 down-regulates vascular endothelial growth factor, adrenomedullin and their receptors and modulates progesterone receptor content in cultured human uterine leiomyoma cells. Hum Reprod. 2006;21(9):2408–2416.
Xu Q, Takekida S, Ohara N, et al. Progesterone receptor modulator CDB-2914 down-regulates proliferative cell nuclear antigen and Bc1-2 protein expression and up-regulates caspase-3 and poly(adenosine 5’-diphosphate-ribose) polymerase expression in cultured human uterine leiomyoma cells. J Clin Endocrinol Metab. 2005;90(2):953–961.
Malik M, Catherino WH. Novel method to characterize primary cultures of leiomyoma and myometrium with the use of confirmatory biomarker gene arrays. Fertil Steril. 2007;87(5):1166–1172.
Pay son M, Malik M, Siti-Nur Morris S, Segars JH, Chason R, Catherino WH. Activating transcription factor 3 gene expression suggests that tissue stress plays a role in leiomyoma development. Fertil Steril. 2009;92(2):748–755.
Malik M, Britten J, Segars J, Catherino WH. Leiomyoma cells in 3-dimensional cultures demonstrate an attenuated response to fas-udil, a rho-kinase inhibitor, when compared to 2-dimensional cultures. Reprod Sci. 2014;21(9):1126–1138.
Catherino W, Salama A, Potlog-Nahari C, Leppert P, Tsibris J, Segars J. Gene expression studies in leiomyomata: new directions for research. Semin Reprod Med. 2004;22(2):83–90.
Barker NM, Carrino DA, Caplan Al, et al. Proteoglycans in leiomyoma and normal myometrium: abundance, steroid hormone control, and implications for pathophysiology. Reprod Sci. 2016;23(3):302–309.
Flake GP, Moore AB, Sutton D, et al. The natural history of uterine leiomyomas: light and electron microscopic studies of fibroid phases, interstitial ischemia, inanosis, and reclamation. Obstet Gynecol Int. 2013;2013:528376.
Bouchard P, Chabbert-Buffet N, Fauser B. Selective progeter-one receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertil Steril. 2011;96(5):1175–1189.
Melis GB, Piras B, Marotto MF, et al. Pharmacokinetic evaluation of ulipristal acetate for uterine leiomyoma treatment. Expert Opin Drug Metab Toxicol. 2012;8(7):901–908.
Chwalisz K, Perez MC, DeManno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26(3):423–438.
Courtoy GE, Donnez J, Marbaix E, Dolmans MM. In vivo mechanisms of uterine myoma volume reduction with ulipristal acetate treatment. Fertil Steril. 2015;104(2):426–434.el.
Dou Q, Tarnuzzer RW, Williams RS, Schultz GS, Chegini N. Differential expression of matrix metalloproteinases and their tissue inhibitors in leiomyomata: a mechanism for gonadotrophin releasing hormone agonist-induced tumour regression. Mol Hum Reprod. 1997;3(11):1005–1014.
Palmer SS, Haynes-Johnson D, Diehl T, Nowak RA. Increased expression of stromelysin 3 mRNA in leiomyomas (uterine fibroids) compared with myometrium. J Soc Gynecol Investig. 1998;5(4):203–209.
Bogusiewicz M, Stryjecka-Zimmer M, Postawski K, Jakimiuk AJ, Rechberger T. Activity of matrix metalloproteinase-2 and -9 and contents of their tissue inhibitors in uterine leiomyoma and corresponding myometrium. Gynecol Endocrinol. 2007;23(9):541–546.
Korompelis P, Piperi C, Adamopoulos C, et al. Expression of vascular endothelial factor-A, gelatinases (MMP-2, MMP-9) and TIMP-1 in uterine leiomyomas. Clin Chem Lab Med. 2015;53(9):1415–1424.
Wolahska M, Sobolewski K, Bahkowski E, Jaworski S. Matrix metalloproteinases of human leiomyoma in various stages of tumor growth. Gynecol Obstet Invest. 2004;58(1):14–18.
Haider SK, Osteen KG. Al-Hendy A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum Reprod. 2013;28(9):2407–2416.
Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7(2):193–203.
Parks WC, Wilson CL. Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617–629.
Zuo F, Kaminski N, Eugui E, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci U S A. 2002;99(9):6292–6297.
Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14(2):163–176.
Dayer C, Stamenkovic I. Recruitment of matrix metalloproteinase-9 (MMP-9) to the fibroblast cell surface by lysyl hydroxylase 3 (LH3) triggers transforming growth factor-P (TGF-P) activation and fibroblast differentiation. J Biol Chem. 2015;290(22):13763–13778.
Ciarmela P, Carrarelli P, Islam MS, et al. Ulipristal acetate modulates the expression and functions of activin a in leiomyoma cells. Reprod Sci. 2014;21(9):1120–1125.
McCarthy-Keith D, Malik M, Britten J, Segars J, Catherino W. Gonadotropin-releasing hormone agonist increases expression of osmotic response genes in leiomyoma cells. Fertil Steril. 2011;95(7):2383–2387.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cox, J., Malik, M., Britten, J. et al. Ulipristal Acetate and Extracellular Matrix Production in Human Leiomyomas In Vivo: A Laboratory Analysis of a Randomized Placebo Controlled Trial. Reprod. Sci. 25, 198–206 (2018). https://doi.org/10.1177/1933719117728802
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117728802