Abstract
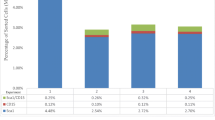
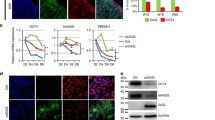
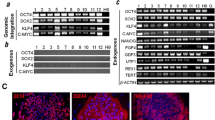
Embryoid bodies (EBs) can serve as a system for evaluating pluripotency, cellular differentiation, and tissue morphogenesis. In this study, we use EBs derived from mouse embryonic stem cells (mESCs) and human amniocyte-derived induced pluripotent stem cells (hAdiPSCs) as a model for ovarian granulosa cell (GC) development and steroidogenic cell commitment. We demonstrated that spontaneously differentiated murine EBs (mEBs) and human EBs (hEBs) displayed ovarian GC markers, such as aromatase (CYPI9AI), FOXL2, AMHR2, FSHR, and GJAI. Comparative microarray analysis identified both shared and unique gene expression between mEBs and the maturing mouse ovary. Gene sets related to gonadogenesis, lipid metabolism, and ovarian development were significantly overrepresented in EBs. Of the 29 genes, 15 that were differentially regulated in steroidogenic mEBs displayed temporal expression changes between embryonic, postnatal, and mature ovarian tissues by polymerase chain reaction. Importantly, both mEBs and hEBs were capable of gonadotropin-responsive estradiol (E2) synthesis in vitro (217-759 pg/mL). Live fluorescence-activated cell sorting-sorted AMHR2+ granulosa-like cells from mEBs continued to produce E2 after purification (15.3 pg/mL) and secreted significantly more E2 than AMHR2 cells (8.6 pg/mL, P <.05). We conclude that spontaneously differentiated EBs of both mESC and hAdiPSC origin can serve as a biologically relevant model for ovarian GC differentiation and steroidogenic cell commitment. These cells should be further investigated for therapeutic uses, such as stem cell-based hormone replacement therapy and in vitro maturation of oocytes.
Similar content being viewed by others
References
Bastings L, Beerendonk CC, Westphal JR, et al. Allotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Hum Reprod Update. 2019;19(5):483–506.
Shaw JM, Bowles J, Koopman P, Wood EC, Trounson AO. Fresh and cryopreserved ovarian tissue samples from donors with lymphoma transmit the cancer to graft recipients. Hum Reprod. 1996; 11(8):1668–73.
van der Stege JG, Groen H, van Zadelhoff SJ, et al. Decreased androgen concentrations and diminished general and sexual well-being in women with premature ovarian failure. Menopause. 2008;15(1):23–31.
Madalinska JB, van Beurden M, Bleiker EM, et al. The impact of hormone replacement therapy on menopausal symptoms in younger high-risk women after prophylactic salpingo-oophorect-omy. J Clin Oncol. 2006;24(22):3576–3582.
Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201.
Henderson VW. Cognitive changes after menopause: influence of estrogen. Clin Obstet Gynecol. 2008;51(3):618–626.
Anasti JN, Kalantaridou SN, Kimzey LM, Defensor RA, Nelson LM. Bone loss in young women with karyotypically normal spon-taneous premature ovarian failure. Obstet Gynecol. 1998;91(1): 12–15.
Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19(14):3306–3311.
Kalantaridou SN, Naka KK, Papanikolaou E, et al. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab. 2004;89(8):3907–3913.
Beral V, Banks E, Reeves G. Evidence from randomised trials on the long-term effects of hormone replacement therapy. Lancet. 2002;360(9337):942–944.
Daly E, Vessey MP, Hawkins MM, Carson JL, Gough P, Marsh S. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348(9033):977–980.
Ross RK, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Lnst. 2000;92(4):328–332.
Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283(4):485–491.
Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85(2):304–313.
Lacey JV Jr, Mink PJ, Lubin JH, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002; 288(3):334–341.
Hubner K, Fuhrmann G, Christenson LK, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003; 300(5623):1251–1256.
Novak I, Lightfoot DA, Wang H, Eriksson A, Mahdy E, Hoog C. Mouse embryonic stem cells form follicle-like ovarian structures but do not progress through meiosis. Stem Cells. 2006;24(8): 1931–1936.
Chen HF, Kuo HC, Chien CL, et al. Derivation, characterization and differentiation of human embryonic stem cells: comparing serum-containing versus serum-free media and evidence of germ cell differentiation. Hum Reprod. 2007;22(2):567–577.
Psathaki OE, Hubner K, Sabour D, et al. Ultrastructural characterization of mouse embryonic stem cell-derived oocytes and granulosa cells. Stem Cells Dev. 2011;20(12):2205–2215.
Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338(6109):971–975.
White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3): 413–421.
Zhang J, Li H, Wu Z, et al. Differentiation of rat iPS cells and ES cells into granulosa cell-like cells in vitro. Acta Biochim Biophys Sin (Shanghai). 2013;45(4):289–295.
Lan CW, Chen MJ, Jan PS, Chen HF, Ho HN. Differentiation of human embryonic stem cells into functional ovarian granulosalike cells. J Clin Endocrinol Metab. 2013;98(9):3713–3723.
Woods DC, White YA, Niikura Y, Kiatpongsan S, Lee HJ, Tilly JL. Embryonic stem cell-derived granulosa cells participate in ovarian follicle formation in vitro and in vivo. Reprod Sci 2013; 20(5):524–535.
Gerami-Naini B, Dovzhenko OV, Durning M, Wegner FH, Thomson JA, Golos TG. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology. 2004;145(4):1517–1524.
Guven S, Lindsey JS, Poudel I, et al. Functional maintenance of differentiated embryoid bodies in microfluidic systems: a platform for personalized medicine. Stem Cells Transl Med. 2015; 4(3):261–268.
Anchan RM, Gerami-Naini B, Lindsey JS, et al. Efficient differentiation of steroidogenic and germ-like cells from epigenetically-related iPSCs derived from ovarian granulosa cells. PLoS One. 2015;10(3):e0119275.
Anchan RM, Quaas P, Gerami-Naini B, et al. Amniocytes can serve a dual function as a source of iPS cells and feeder layers. Hum Mol Genet. 2011;20(5):962–974.
Du P, Kibbe WA, Lin SM. Lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24(13): 1547–1548.
Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3.
Sassoon D, Rosenthal N. Detection of messenger RNA by in situ hybridization. Methods Enzymol. 1993;225:384-404.
Narod SA. Hormone replacement therapy and the risk of breast cancer. Nat Rev Clin Oncol. 2011;8(11):669–676.
Beral V, Reeves G, Bull D, Green J, Million Women Study C. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103(4):296–305.
Hovatta O. Methods for cryopreservation of human ovarian tissue. Reprod Biomed Online. 2005;10(6):729–734.
Shaw J, Trounson A. Oncological implications in the replacement of ovarian tissue. Hum Reprod. 1997;12(3):403–405.
Uda M, Ottolenghi C, Crisponi L, et al. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle develop-ment. Hum Mol Genet. 2004;13(11): 1171–1181.
Ottolenghi C, Omari S, Garcia-Ortiz JE, et al. Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet. 2005; 14(14):2053–2062.
Yoon SJ, Kim KH, Chung HM, et al. Gene expression profiling of early follicular development in primordial, primary, and secondary follicles. Fertil Steril. 2006;85(1):193–203.
Dharma SJ, Modi DN, Nandedkar TD. Gene expression profiling during early folliculogenesis in the mouse ovary. Fertil Steril. 2009;91(suppl 5):2025–2036.
Hasegawa A, Kumamoto K, Mochida N, Komori S, Koyama K. Gene expression profile during ovarian folliculogenesis. J Reprod Immunol. 2009;83(1-2):40–44.
Irie N, Weinberger L, Tang WW, et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell. 2015;160(1–2): 253–268.
Jadhav U, Jameson JL. Steroidogenic factor-1 (SF-1)-driven differentiation of murine embryonic stem (ES) cells into a gonadal lineage. Endocrinology. 2011;152(7):2870–2882.
Choate JV, Resko JA. Paradoxical effect of an aromatase inhibitor, CGS 20267, on aromatase activity in guinea pig brain. J Steroid Biochem Mol Biol. 1996;58(4):411–415.
Darabi R, Gehlbach K, Bachoo RM, et al. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med. 2008;14(2): 134–143.
Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the anterior hypothalamus of adult male and female rats. Endocrinology. 1972;90(1):295–298.
Sasano H, Uzuki M, Sawai T, et al. Aromatase in human bone tissue. J Bone Miner Res. 1997;12(9):1416–1423.
Meseguer A, Puche C, Cabero A. Sex steroid biosynthesis in white adipose tissue. Horm Metab Res. 2002;34(11-12): 731–736.
Belanger C, Luu-The V, Dupont P, Tchernof A. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm Metab Res. 2002;34(11–12):737–745.
Niikura Y, Niikura T, Tilly JL. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following trans-plantation into a young host environment. Aging (Albany NY). 2009;1(12):971–978.
Aflatoonian B, Ruban L, Jones M, Aflatoonian R, Fazeli A, Moore HD. In vitro post-meiotic germ cell development from human embryonic stem cells. Hum Reprod. 2009;24(12): 3150–3159.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lipskind, S., Lindsey, J.S., Gerami-Naini, B. et al. An Embryonic and Induced Pluripotent Stem Cell Model for Ovarian Granulosa Cell Development and Steroidogenesis. Reprod. Sci. 25, 712–726 (2018). https://doi.org/10.1177/1933719117725814
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117725814