Abstract
Objective
Ovarian tissue cryopreservation and transplantation are the only options accepted for prepubertal girls and women requiring immediate chemotherapy. Ischemia–reperfusion injury is the main obstacle for ovarian tissue transplantation. In the present study, we aimed to evaluate the effects of recombinant human erythropoietin (EPO) on tissue viability in autotransplanted rat ovaries.
Study Design
Seventeen female rats were randomized into 3 groups as sham control group (n = 5), EPO-treated group (n = 6), and EPO-untreated group (n = 6). Both ovaries were excised and transplanted into a subcutaneous pouch formed at the anterior abdominal wall in the EPO-treated and untreated groups. In the EPO group, 5000 U/kg EPO was applied as local injection to the site that ovarian tissue was placed and the dose was repeated with the same route at the end of the fourth week. After 2 months, ovaries were removed and blood samples were obtained. Levels of estradiol (E2), vascular endothelial growth factor (VEGF), VEGF-C, and lipid hydroperoxidase (LPO) and the activity of glutathione peroxidase (GPX), superoxide dismutase (SOD), and catalase (CAT) were measured both in blood and tissue samples. Histopathological and morphometric analyses were also performed on tissue samples.
Results
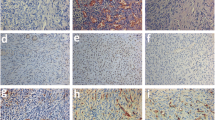
Considering serum levels, mean CAT was significantly higher (P =.003) and mean SOD (P =.033), LPO (P =.050), VEGF (P =.001), and VEGF-C (P =.024) were significantly lower in the EPO-treated group than in the untreated group. Mean serum GPX levels were similar. Significantly higher levels of E2 were determined in the EPO group than in the untreated group. Highest serum E2 levels were found in the sham group (P =.001). Tissue levels of GPX (1.23) and CAT (53.17) were significantly higher in the EPO group (P =.002 and P =.001, respectively). However, tissue levels of SOD and LPO, VEGF, and VEGF-C levels were significantly lower in the EPO group than those in the untreated group (P =.033, P =.050, P =.002, and P =.003, respectively). In tissue examination, the highest values of x, y axis and epithelial height were in the sham group. Mean value of the EPO group was found statistically significantly higher than that of the untreated group (P ≤.05). In terms of antral follicle count, ordering was found as sham > EPO-treated > EPO-untreated group. Follicle counts in the EPO group were significantly higher than those in the untreated group (P ≤ 0.05).
Conclusion
Erythropoietin improved the survival of follicles in ovarian grafts most likely by reducing ischemic injury, by improving neoangiogenesis, and by its antioxidant effects.
Similar content being viewed by others
References
Blatt J. Pregnancy outcome in long-term survivors of childhood cancer. Med Pediatr Oncol. 1999;33(1):29–33. doi:10.1002/(SICI)1096-911X199907)33:13.0.CO;2-2
Donnez J, Matinez-Madrid B, Jadoul P, et al. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12(5):519–535. doi:10.1093/humupd/dml032
Donnez J, Bassil S. Indications for cryopreservation of ovarian tissue. Hum Reprod Update. 1998;4(3):248–259. doi:10.1093/humupd/4.3.248
Meirow D, Ben Yehuda D, Pius D, et al. Ovarian tissue banking in patients with Hodgkin’s disease: is safe? Fertil Steril. 1998;69(6): 996–998. doi:10.1016/S0015-0282(98)00093-4
Oktay K, Newton H, Aubard Y, Salha O, Gosden RG. Cryopreservation of immature human oocytes and ovarian tissue: an emerging technology? Fertil Steril. 1998;69(1):1–7. doi:10.1016/S0015-0282(97)00207-0
Donnez J, Godin PA, Qu J, Nisolle M. Gonadal cryopreservation in young patient with gynaecological malignancy. Curr Opin Obstet Gynecol. 2000;12(1):1–9. doi:10.1097/00001703-200002000-00001
Donnez J, Dolmans MM, Matinez-Madrid B, Demylle D, Van Langendonckt A. The role of cryopreservation for women prior to treatment of malignancy. Curr Opin Obstet Gynecol. 2005;17(4):333–338. doi:10.1097/01.gco.0000175348.72566.47
Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15(6):649–665. doi:10.1093/humupd/dmp021
Hemadi M, Abolhassani F, Akbari M, et al. Melatonin promotes the cumulus-oocyte complexes quality of vitrified-thawed murine ovaries; with increased mean number of follicles survival and ovary size following heterotopic transplantation. Eur J Pharmacol. 2009;618(1-3):84–90. doi:10.1016/j.ejphar.2009.07.018
Karaca M, Odabasoglu F, Kumtepe Y, Albayrak A, Cadirci E, Keles ON. Protective effects of erythropoietin on ischemia/reperfusion injury of rat ovary. Eur J Obstet Gynecol Reprod Biol. 2009;144(2):157–162. doi:10.1016/j.ejogrb.2009.03.011
Aubard Y, Piver P, Cognie Y, Fermeaux V, Poulin N, Driancourt MA. Orthotopic and heterotopic autografts of frozen-thawed ovarian cortex in sheep. Hum Reprod. 1999;14(8):2149–2154. doi:10.1093/humrep/14.8.2149
Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at —196°C. Endocrinology. 1999;140(1):462–471. doi:10.1210/en.140.1.462
Oktay K, Newton H, Gosden RG. Transplantation of cryopreserved human ovarian tissue results in follicle growth initiation in SCID mice. Fertil Steril. 2000;73(3):599–603. doi:10.1016/S0015-0282(99)00548-8
Liu J, Van den Elst J, Van den Broecke R, Dhont M. Early massive follicle loss and apoptosis in heterotopically grafted newborn mouse ovaries. Hum Reprod. 2002;17(3):605–611. doi:10.1093/humrep/17.3.605
Israely T, Dafni H, Granot D, Nevo N, Tsafriri A, Neeman M. Vascular remodeling and angiogenesis in ectopic ovarian transplants: a crucial role of pericytes and vascular smooth muscle cells in maintenance of ovarian grafts. Biol Reprod. 2003;68(6): 2055–2064. doi:10.1095/biolreprod.102.011734
Mehranjani MS, Noorafshan A, Hamta A, et al. Effects of vitamin E on ovarian tissue of rats following treatment with p-nonylphenol: a stereological study. Iranian J Reprod Med. 2010:8(1):81–89.
Sayyah-Melli M, Kazemi-Shishvan M, Solaimani-Rad J, et al. The ovario-protective effect of erythropoietin against oxidative damage associated with reperfusion following ovarian torsion in rat. Am J Anim Vet Sci. 2011;6(1):18–24. doi:10.3844/ajavsp.2011.18.24
Commin L, Buff S, Rosset E, et al. Follicle development in cryo-preserved bitch ovarian tissue grafted to immunodeficient mouse. Reprod Fertil Dev. 2012;24(3):461–471. doi:10.1071/RD11166
Wang Y, Chang Q, Sun J, et al. Effects of HMG on revascularization and follicular survival in heterotopic autotransplants of mouse ovarian tissue. Reprod Biomed Online. 2012;24(6):646–653. doi:10.1016/j.rbmo.2012.02.025
Suzuki H, Ishijima T, Maruyama S, Yanagimoto Ueta Y, Abe Y, Saitoh H. Beneficial effect of desialylated erythropoietin administration on the frozen-thawed canine ovarian xenotransplantation. J Assist Reprod Genet. 2008;25(11-12):571–575. doi:10.1007/s10815-008-9271-9
Yang H, Lee HH, Lee HC, Ko DS, Kim SS. Assessment of vascular endothelial growth factor expression and apoptosis in the ovarian graft: can exogenous gonadotropin promote angiogenesis after ovarian transplantation? Fertil Steril. 2008;90(suppl 4):1550–1558. doi:10.1016/j.fertnstert.2007.08.086
Bakan V, Ciralik H, Tolun FI, Atli Y, Mil A, Ozturk S. Protective effect of erythropoietin on torsion/detorsion injury in rat model. J Pediatr Surg. 2009;44(10):1988–1994. doi:10.1016/j.jpedsurg.2009.02.071
Kolusari A, Kamaci M, Zeteroglu S, Altunay H, Sahin HG. Protective effects of erythropoietin on ischemia-reperfusion model in rat ovary. Turkye Klinikleri J Med Sci. 2010;30(4):1189–1195. doi:10.5336/medsci.2009-13553
Lowry O, Rsenbraugh N, Farr L, Randall R. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193(1):265–275.
Ishijima T, Kobayashi Y, Lee DS, et al. Cryopreservation of canine ovaries by vitrification. J Reprod Dev. 2006;52(2): 293–299. doi:10.1262/jrd.17080
Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrere S, Donnez J. Freezing-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82(5):1390–1394. doi:10.1016/j.fertnstert.2004.06.036
Maltaris T, Beckmann MW, Binder H, et al. The effect of a GnRH agonist on cryopreserved human ovarian graft in severe combined immunodeficient mice. Reproduction. 2007;133(2):503–509. doi:10.1530/REP-06-0061
Maltaris T, Dimmler A, Muller A, et al. The use of an openfreezing system with self-seeding for cryopreservation of mouse ovarian tissue. Reprod Domest Anim. 2005;40(3):250–254. doi:10. 1111/j.1439-0531.2005.00595.x
Suzuki H, Ishijima T, Maruyama S, Ueta YY, Abe Y, Saitoh H. Beneficial effects of desialylated erythropoietin administration on the frozen-thawed canine ovarian xenotransplantation. J Assist Reprod Genet. 2008;25:571–575. doi:10.1007/s10815-008-9271-9
Mahmoodi M, Mehranjani MS, Shariatzadeh SMA, Eimani H, Shahverdi A. Effects of erythropoietin on ischemia, follicular survival, and ovarian function in ovarian grafts. Reproduction. 2014;147(5):733–741.
Clarijs R, Schalkwijk L, Ruiter DJ, de Waal RM. Lack of lymphangiogenesis despite coexpression of VEGF-C and its receptor Flt-4 in uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42(7):1422–1428.
Kukk E, Lymboussaki A, Taira S, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122(12):3829–3837.
Muders M, Zhang H, Wang E, Tindall DJ, Datta K. Vascular Endothelial Growth Factor-C protects prostate cancer cells from oxidative stress by the activation of mTORC-2 and AKT-1. Cancer Res. 2009;69(15): 6042–6048.
Skobe M, Hawighorst T, Jackson DG, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7(2):192–198.
Mattila MM, Ruohola JK, Karpanen T, Jackson DG, Alitalo K, Harkonen PL. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int J Cancer. 2002;98(6):946–951.
Cohen B, Addadi Y, Sapoznik S, et al. Transcriptional regulation of vascular endothelial growth factor C by oxidative and thermal stress is mediated by lens epithelium-derived growth factor/p75. Neoplasia. 2009;11(9):921–931.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kolusari, A., Okyay, A.G. & Koçkaya, E.A. The Effect of Erythropoietin in Preventing Ischemia-Reperfusion Injury in Ovarian Tissue Transplantation. Reprod. Sci. 25, 406–413 (2018). https://doi.org/10.1177/1933719117715127
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117715127