Abstract
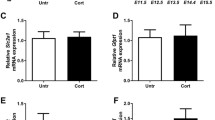
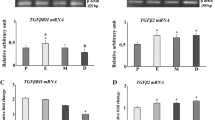
The incidence of diabetes mellitus for young people rises since years. A preconceptional diabetes mellitus leads to subfertility. Most of the causes for a diabetic subfertility are still unknown. Stress can significantly deteriorate glycemic control in diabetes. Several mechanisms by which “stress hormones”, like adrenaline and Cortisol or corticosterone, can contribute to the regulation of glucose homeostasis have been identified. Using reverse transcription-polymerase chain reaction (RT-PCR) and quantitative real-time RT-PCR, we examined the expression of adrenergic receptors and the glucocorticoid receptor transcripts in the female rabbit reproductive tract and in gastrulating blastocysts developed in normoinsulinemic mothers and in mothers with experimentally induced diabetes mellitus type I. The glucocorticoid receptor expression was detected in the reproductive tract as well as in gastrulating blastocysts at a high level. In maternal endometrium, αID-, α2A-, βl-, and β2-adrenergic receptors were expressed, whereby β1 transcript was not detectable in the endometrium from diabetic mothers. In preimplantation embryos, all 9 adrenergic receptors were expressed, most of them predominantly in the embryoblast. A maternal diabetes mellitus altered α2A-adrenergic receptor expression in the blastocyst and reversed the ratio of α2A transcript quantity between embryoblast and trophoblast. Our results show that the maternal reproductive tract and the preimplantation embryo express a distinct pattern of the stress response system. Alterations in the pattern and/or in functionality are likely linked to subfertility in diabetes mellitus.
Similar content being viewed by others
References
International Diabetes Federation. IDF Diabetes Atlas. 7th ed. Brussels, Belgium: International Diabetes Federation, 2015. ISBN:978-2.930229-81-2.
Vehik K, Hamman RF, Lezotte D, et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30(3):503–509. doi:10.2337/dc06-1837.
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053.
Deutscher Gesundheitsbericht 2015. Kirchheim + Co GmbH. ISSN 1614-824X.
Jonasson JM, Brismar K, Sparen P, et al. Fertility in women with type 1 diabetes: a population-based cohort study in Sweden. Diabetes Care. 2007;30(9):2271–2276. doi:10.2337/dc06-2574.
Rondo PHC, Ferreira RF, Nogueira F, Ribeiro MCN, Lobert H, Artes R. Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. Eur J Clin Nutr. 2003;57(2):266–272. doi:10.1038/sj.ejcn.1601526.
Faisal-Cury A, Rossi Menezes P. Prevalence of anxiety and depression during pregnancy in a private setting sample. Arch Womens Merit Health. 2007;10(1):25–32. doi:10.1007/s00737-006-0164-6.
Maeng LY, Milad MR. Sex differences in anxiety disorders: interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106–117. doi:10.1016/j.yhbeh.2015.04.002.
Surwit RS, Schneider MS. Role of stress in the etiology and treatment of diabetes mellitus. Psychosom Med. 1993;55(4):380–393.
Wales JK. Does psychological stress cause diabetes? Diabet Med J Br Diabet Assoc. 1995;12(2):109–112.
Lloyd C, Smith J, Weinger K. Stress and diabetes: a review of the links. Diabetes Spectr. 2005;18(2):121–127. doi:10.2337/diaspect.18.2.121.
Boschmann M, Krupp G, Luft FC, Klaus S, Jordan J. In vivo response to alpha(1)-adrenoreceptor stimulation in human white adipose tissue. Obes Res. 2002;10(6):555–558. doi:10.1038/oby. 2002.75.
Flechtner-Mors M, Jenkinson CP, Alt A, Biesalski HK, Adler G, Ditschuneit HH. Sympathetic regulation of glucose uptake by the alphal-adrenoceptor in human obesity. Obes Res. 2004;12(4):612–620. doi:10.1038/oby.2004.70.
Dehvari N, Hutchinson DS, Nevzorova J, et al. P(2)-Adrenocep-tors increase translocation of GLUT4 via GPCR kinase sites in the receptor C-terminal tail. Br J Pharmacol. 2012;165(5):1442–1456. doi:10.1111/J.1476-5381.2011.01647.X.
Hunt DG, Ding Z, Ivy JL. Clenbuterol prevents epinephrine from antagonizing insulin-stimulated muscle glucose uptake. J Appl Physiol (1985). 2002;92(3):1285–1292. doi:10.1152/japplphysiol.01009.2001.
Fagerholm V, Haaparanta M, Scheinin M. α2-adrenoceptor regulation of blood glucose homeostasis. Basic Clin Pharmacol Toxicol. 2011;108(6):365–370. doi: 10.1111/j.1742-7843.2011.00699.x.
Hamamdzic D, Duzic E, Sherlock JD, Lanier SM. Regulation of alpha 2-adrenergic receptor expression and signaling in pancreatic beta-cells. Am J Physiol. 1995;269(1 pt 1):E162–E171.
Jungheim ES, Moley KH. The impact of type 1 and type 2 diabetes mellitus on the oocyte and the preimplantation embryo. Semin Reprod Med. 2008;26(2):186–195. doi:10.1055/s-2008-1042957.
Ramin N, Thieme R, Fischer S, et al. Maternal diabetes impairs gastrulation and insulin and IGF-I receptor expression in rabbit blastocysts. Endocrinology. 2010;151(9):4158–4167. doi:10. 1210/en.2010-0187.
Cikos S, Veselá J, Il’ková G, Rehák P, Czikkova S, Koppel J. Expression of beta adrenergic receptors in mouse oocytes and preimplantation embryos. Mol Reprod Dev. 2005;71(2):145–153. doi:10.1002/mrd.20256.
Cikos S, Rehák P, Czikková S, Veselá J, Koppel J. Expression of adrenergic receptors in mouse preimplantation embryos and ovulated oocytes. Reproduction. 2007;133(6):1139–1147. doi:10.1530/REP-07-0006.
Burkuš J, Cikoš S, Fabian D, Kubandová J, Czikková S, Koppel J. Maternal restraint stress negatively influences growth capacity of preimplantation mouse embryos. Gen Physiol Biophys. 2013;32(1):129–137. doi:10.4149/gpb_2013010.
Burkuš J, Kačmarová M, Kubandová J, et al. Stress exposure during the preimplantation period affects blastocyst lineages and offspring development. J Reprod Dev. 2015;61(4):325–331. doi: 10.1262/jrd.2015-012.
Čikoš Š, Czikková S, Chrenek P, et al. Expression of adrenergic receptors in bovine and rabbit oocytes and preimplantation embryos. Reprod Domest Anim Zuchthyg. 2014;49(1):92–100. doi: 10.1111/rda.12233.
Siemieniuch MJ, Majewska M, Takahashi M, et al. Are glucocorticoids auto- and/or paracrine factors in early bovine embryo development and implantation? Reprod Biol. 2010;10(3):249–256.
Schindler M, Fischer S, Thieme R, Fischer B, Santos AN. cAMP-responsive element binding protein: a vital link in embryonic hormonal adaptation. Endocrinology. 2013;154(6):2208–2221. doi:10.1210/en.2012-2096.
Fischer B, Chavatte-Palmer P, Viebahn C, Navarrete Santos A, Duranthon V. Rabbit as a reproductive model for human health. Reproduction. 2012;144(1):1–10. doi:10.1530/REP-12-0091.
Santos AN, Körber S, Küllertz G, Fischer G, Fischer B. Oxygen stress increases prolyl cis/trans isomerase activity and expression of cyclophilin 18 in rabbit blastocysts. Biol Reprod. 2000;62(1):1–7.
Gürke J, Schindler M, Pendzialek SM, et al. Maternal diabetes promotes mTORC1 downstream signalling in rabbit preimplantation embryos. Reproduction. 2016;151(5):465–476. doi:10.1530/REP-15-0523.
Higgins DG, Thompson JD, Gibson TJ. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 1996;266:383–402. doi: 10.1016/S0076-6879(96)66024-8.
Altschul SF, Madden TL. Schäffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402.
Thieme R, Schindler M, Ramin N, et al. Insulin growth factor adjustment in preimplantation rabbit blastocysts and uterine tissues in response to maternal type 1 diabetes. Mol Cell Endocrinol. 2012;358(1):96–103.doi:10.1016/j.mce.2012.03.007.
Mamo S, Gal AB, Polgar Z, Dinnyes A. Expression profiles of the pluripotency marker gene POU5F1 and validation of reference genes in rabbit oocytes and preimplantation stage embryos. BMC Mol Biol. 2008;9:67. doi:10.1186/1471-2199-9-67.
Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7): RESEARCH0034.
Cikos S, Koppel J. Transformation of real-time PCR fluorescence data to target gene quantity. Anal Biochem. 2009;384(1):1–10. doi:10.1016/j.ab.2008.08.031.
Tica AA, Dun E, Tica V, Cojocaru V, Tica OS, Berceanu S. The autonomic innervation of the uterus: a short review on pharmacological aspects. Gineco.ro. 2011;7(2):86–91.
Chen Q, Zhang Y, Peng H, et al. Transient α2-adrenoceptor activation confers pregnancy loss by disrupting embryo spacing at implantation. J Biol Chem. 2011;286(6):4349–4356. doi:10.1074/jbc.M110.197202.
Bruzzone ME, Fabres C, Benitez DA, Castellon EA. Zegers-Hochschild F. Influence of embryonic conditioned media upon the endometrial beta-adrenergic receptor. Reprod Biomed Online. 2005;11(1):58–63.
Altan VM, Arioglu E, Guner S, Ozcelikay AT. The influence of diabetes on cardiac beta-adrenoceptor subtypes. Heart Fail Rev. 2007;12(1):58–65. doi:10.1007/sl0741-007-9005-6.
Safi SZ, Qvist R, Yan GOS, Ismail ISB. Differential expression and role of hyperglycemia induced oxidative stress in epigenetic regulation of β1, β2 and β3-adrenergic receptors in retinal endothelial cells. BMC Med Genomics. 2014;7:29. doi:10.1186/1755-8794-7-29.
Zhao X, Zhang Y, Leander M, Li L, Wang G, Emmett N. Altered expression profile of renal α(1D)-adrenergic receptor in diabetes and its modulation by PPAR agonists. J Diabetes Res. 2014;2014: 725634. doi:10.1155/2014/725634.
Weber LP, Macleod KM. Influence of streptozotocin diabetes on the alpha-1 adrenoceptor and associated G proteins in rat arteries. J Pharmacol Exp Ther. 1997;283(3):1469–1478.
Kobayashi T, Matsumoto T, Ooishi K, Kamata K. Differential expression of alpha2D-adrenoceptor and eNOS in aortas from early and later stages of diabetes in Goto-Kakizaki rats. Am J Physiol Heart Circ Physiol. 2004;287(1):H135–H143. doi: 10.1152/ajpheart.01074.2003.
Brede M, Philipp M, Knaus A, Muthig V, Hein L. alpha2-adrenergic receptor subtypes—novel functions uncovered in gene-targeted mouse models. Biol Cell. 2004;96(5):343–348. doi: 10.1016/j.biolcel.2004.03.006.
Khatchadourian C, Menezo Y, Gerard M, Thibault C. Catecholamines within the rabbit oviduct at fertilization time. Hum Reprod. 1987;2(1):1–5.
Way AL, Barbato GF, Killian GJ. Identification of norepinephrine in bovine oviductal fluid by high performance liquid chromatography. Life Sci. 2001;70(5):567–576.
Bodis J, Hartmann G, Tinneberg HR, et al. Relationship between the monoamine, progesterone and estradiol content in follicular fluid of preovulatory Graafian follicles after superovulation treatment. Gynecol Obstet Invest. 1993;35(4):232–235.
Pendleton RG, Rasheed A, Roychowdhury R, Hillman R. A new role for catecholamines: ontogenesis. Trends Pharmacol Sci. 1998;19(7):248–251.
Weiss ER, Maness P, Lauder JM. Why do neurotransmitters act like growth factors? Perspect Dev Neurobiol. 1998;5(4):323–335.
Herlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Hum Dev. 2001;65(1):21–37.
Duzic E, Lanier SM. Factors determining the specificity of signal transduction by guanine nucleotide-binding protein-coupled receptors. III. Coupling of alpha 2-adrenergic receptor subtypes in a cell type-specific manner. J Biol Chem. 1992;267(33):24045–24052.
Eason MG, Liggett SB. Identification of a Gs coupling domain in the amino terminus of the third intracellular loop of the alpha 2A-adrenergic receptor. Evidence for distinct structural determinants that confer Gs versus Gi coupling. J Biol Chem. 1995;270(42):24753–24760.
Pohjanoksa K, Jansson CC, Luomala K, Marjamäki A, Savola JM, Scheinin M. Alpha2-adrenoceptor regulation of adenylyl cyclase in CHO cells: dependence on receptor density, receptor subtype and current activity of adenylyl cyclase. Eur J Pharmacol. 1997;335(1):53–63.
Mhaouty S, Cohen-Tannoudji J, Bouet-Alard R, Limon-Boulez I, Maltier JP, Legrand C. Characteristics of the alpha 2/beta 2-adrenergic receptor-coupled adenylyl cyclase system in rat myometrium during pregnancy. J Biol Chem. 1995;270(18):11012–11016.
Xu J, He J, Castleberry AM, Balasubramanian S, Lau AG, Hall RA. Heterodimerization of alpha 2A- and beta 1-adrenergic receptors. J Biol Chem. 2003;278(12):10770–10777. doi:10.1074/jbc.M207968200.
Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132(5):1033–1044. doi:10.1016/j.jaci.2013.09.007.
Michael AE, Papageorghiou AT. Potential significance of physiological and pharmacological glucocorticoids in early pregnancy. Hum Reprod Update. 2008;14(5):497–517. doi:10.1093/humupd/dmn021.
Nepomnaschy PA, Welch KB, McConnell DS, Low BS. Strass-mann BI, England BG. Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci U S A. 2006;103(10):3938–3942. doi:10.1073/pnas.0511183103.
Lewicka S, von Hagens C, Hettinger U, et al. Cortisol and cortisone in human follicular fluid and serum and the outcome of IVF treatment. Hum Reprod. 2003;18(8):1613–1617.
Lamy J, Liere P, Pianos A, Aprahamian F, Mermillod P, Saint-Dizier M. Steroid hormones in bovine oviductal fluid during the estrous cycle. Theriogenology. 2016;86(6):1409–1420. doi:10. 1016/j.theriogenology.2016.04.086.
Johnson DC, Dey SK. Role of histamine in implantation: dexa-methasone inhibits estradiol-induced implantation in the rat. Biol Reprod. 1980;22(5):1136–1141.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seeling, T., Čikoš, Š., Grybel, K.J. et al. A Diabetic Pregnancy Alters the Expression of Stress-Related Receptors in Gastrulating Rabbit Blastocyst and in the Reproductive Tract. Reprod. Sci. 25, 174–184 (2018). https://doi.org/10.1177/1933719117707055
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117707055