Abstract
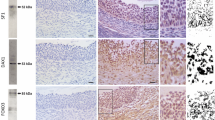
Prenatal testosterone (T)-treated sheep, similar to women with polycystic ovary syndrome (PCOS), manifests reproductive defects that include multifollicular ovarian phenotype. Women with PCOS manifest increased ovarian matrix metalloproteinases (MMPs) activity. We tested the hypothesis that gestational T excess in sheep would alter ovarian expression of MMPs, tissue inhibitors of MMP (TIMP) and their target proteins laminin B (LAMB), collagen, tumor necrosis factor alpha (TNF), and connexin 43 (GJA1) consistent with increased MMP activity and that these changes are developmentally regulated. The ovarian content of these proteins was quantified by immunohistochemistry in fetal day 90, 140, and adult (21 months of age) ovaries. Prenatal T excess lowered GJA1 protein content in stroma and granulosa cells of primary follicles from fetal day 90 ovaries and decreased stromal MMP9, TIMP1, and LAMB in fetal day 140 ovaries. In the adult, prenatal T-treatment (1) increased MMP9 in theca cells of large preantral follicles and stroma, TNF in granulosa cells of small and large preantral follicles and theca cells of large preantral and antral follicles, and GJA1 in stroma, theca cells of large preantral follicles, and granulosa cells of antral follicles and (2) reduced TIMP1 in stroma, theca cells of large preantral and antral follicles, LAMB in stroma and small prenatral follicles, and collagen content in stroma and around antral follicles. These findings suggest a net increase in MMP activity and its target proteins TNF and GJA1 in prenatal T-treated adult but not in fetal ovaries and their potential involvement in the development of multifollicular morphology.
Similar content being viewed by others
References
Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525.
Conway G, Dewailly D, Diamanti-Kandarakis E, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171(4): P1-P29.
Abbott DH, Barnett DK, Levine JE, et al. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod. 2008;79(1): 154–163.
Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013;78(8): 734–740.
Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373(1-2):8–20.
Maliqueo M, Benrick A, Stener-Victorin E. Rodent models of polycystic ovary syndrome: phenotypic presentation, pathophysiology, and the effects of different interventions. Semin Reprod Med. 2014;32(3):183–193.
Cardoso RC, Puttabyatappa M, Padmanabhan V. Steroidogenic versus metabolic programming of reproductive neuroendocrine, ovarian and metabolic dysfunctions. Neuroendocrinology. 2015; 102(3):226–237.
Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146(7):3185–3193.
Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects: partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147(4):1997–2007.
Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10(2): 107–117.
Franks S, Mason H, Willis D. Follicular dynamics in the polycystic ovary syndrome. Mol Cell Endocrinol. 2000;163(1-2): 49–52.
Dumesic DA, Richards JS. Ontogeny of the ovary in polycystic ovary syndrome. Fertil Steril. 2013;100(1):23–38.
Steckler TL, Lee JS, Ye W, Inskeep EK, Padmanabhan V. Developmental programming: exogenous gonadotropin treatment rescues ovulatory function but does not completely normalize ovarian function in sheep treated prenatally with testosterone. Biol Reprod. 2008;79(4):686–695.
Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137(5):865–877.
Padmanabhan V, Salvetti NR, Matiller V, Ortega HH. Developmental programming: prenatal steroid excess disrupts key members of intraovarian steroidogenic pathway in sheep. Endocrinology. 2014;155(9):3649–3660.
Ortega HH, Rey F, Velazquez MM, Padmanabhan V. Developmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheep. Biol Reprod. 2010;82(6):1065–1075.
Veiga-Lopez A, Ye W, Padmanabhan V. Developmental programming: prenatal testosterone excess disrupts anti-Mu¨ llerian hormone expression in preantral and antral follicles. Fertil Steril. 2012;97(3):748–756.
Salvetti NR, Ortega HH, Veiga-Lopez A, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on ovarian cell proliferation and apoptotic factors in sheep. Biol Reprod. 2012;87(1):22, 1–10.
Curry TE Jr, Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med. 2006;24(4):228–241.
Curry TE Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24(4):428–465.
Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–21494.
Baka S, Zourla K, Kouskouni E, et al. Matrix metalloproteinases 2 and 9 and their tissue inhibitors in the follicular fluid of patients with polycystic ovaries undergoing in vitro fertilisation. In Vivo. 2010;24(3):293–296.
Lahav-Baratz S, Kraiem Z, Shiloh H, Koifman M, Ishai D, Dirnfeld M. Decreased expression of tissue inhibitor of matrix metalloproteinases in follicular fluid from women with polycystic ovaries compared with normally ovulating patients undergoing in vitro fertilization. Fertil Steril. 2003;79(3):567–571.
Peng HJ, Dai DZ, Ji H, Dai Y. The separate roles of endothelin receptors participate in remodeling of matrix metalloproteinase and connexin 43 of cardiac fibroblasts in maladaptive response to isoproterenol. Eur J Pharmacol. 2010;634(1-3): 101–106.
Wu X, Huang W, Luo G, Alain LA. Hypoxia induces connexin 43 dysregulation by modulating matrix metalloproteinases via MAPK signaling. Mol Cell Biochem. 2013;384(1-2):155–162.
Gittens JE, Mhawi AA, Lidington D, Ouellette Y, Kidder GM. Functional analysis of gap junctions in ovarian granulosa cells: distinct role for connexin43 in early stages of folliculogenesis. Am J Physiol Cell Physiol. 2003;284(4):C880–C887.
Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction. 2002;123(5):613–620.
Winterhager E, Kidder GM. Gap junction connexins in female reproductive organs: implications for women’s reproductive health. Hum Reprod Update. 2015;21(3):340–352.
Gearing AJ, Beckett P, Christodoulou M, et al. Matrix metalloproteinases and processing of pro-TNF-alpha. J Leukoc Biol. 1995;57(5):774–777.
Jiang JY, Cheung CK, Wang Y, Tsang BK. Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front Biosci. 2003;8:d222–d237.
Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991;88(20):9292–9296.
Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007;148(7):3532–3540.
Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep. Biol Reprod. 2009;80(4):726–736.
Ortega HH, Veiga-Lopez A, Sreedharan S, et al. Developmental programming: does prenatal steroid excess disrupt the ovarian VEGF system in sheep? Biol Reprod. 2015;93(3):58.
Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89(5):397–410.
Lejeune M, Jaen J, Pons L, et al. Quantification of diverse subcellular immunohistochemical markers with clinicobiological relevancies: validation of a new computer-assisted image analysis procedure. J Anat. 2008;212(6):868–878.
Rasband W. Quantifying stained liver tissue. 2016; https://imagej.nih.gov/ij/docs/examples/stained-sections/index.html. Updated March 16, 2011; Accessed November 03, 2016.
Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159.
Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82(4):591–605.
Padmanabhan V, Veiga-Lopez A, Herkimer C, et al. Developmental programming: prenatal and postnatal androgen antagonist and insulin sensitizer interventions prevent advancement of puberty and improve LH surge dynamics in prenatal testosteronetreated sheep. Endocrinology. 2015;156(7):2678–2692.
Monniaux D, Huet C, Besnard N, et al. Follicular growth and ovarian dynamics in mammals. J Reprod Fertil Suppl. 1997;51:3–23.
Rodgers RJ, Irving-Rodgers HF, Russell DL. Extracellular matrix of the developing ovarian follicle. Reproduction. 2003;126(4): 415–424.
Bagavandoss P. Differential distribution of gelatinases and tissue inhibitor of metalloproteinase-1 in the rat ovary. J Endocrinol. 1998;158(2):221–228.
Garcia R, Ballesteros LM, Hernandez-Perez O, et al. Metalloproteinase activity during growth, maturation and atresia in the ovarian follicles of the goat. Anim Reprod Sci. 1997;47(3): 211–228.
Vos MC, van der Wurff AA, Last JT, et al. Immunohistochemical expression of MMP-14 and MMP-2, and MMP-2 activity during human ovarian follicular development. Reprod Biol Endocrinol. 2014;12:12.
Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002;66(4):1134–1150.
Veiga-Lopez A, Steckler TL, Abbott DH, et al. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84(1):87–96.
Strott CA, Sundel H, Stahlman MT. Maternal and fetal plasma progesterone, cortisol, testosterone and 17beta-estradiol in preparturient sheep: response to fetal ACTH infusion. Endocrinology. 1974;95(5):1327–1339.
Hauger RL, Karsch FJ, Foster DL. A new concept for control of the estrous cycle of the ewe based on the temporal relationships between luteinizing hormone, estradiol and progesterone in peripheral serum and evidence that progesterone inhibits tonic LH secretion. Endocrinology. 1977;101(3):807–817.
Osteen KG, Keller NR, Feltus FA, Melner MH. Paracrine regulation of matrix metalloproteinase expression in the normal human endometrium. Gynecol Obstet Invest. 1999;48(suppl 1):2–13.
Rawdanowicz TJ, Hampton AL, Nagase H, Woolley DE, Salamonsen LA. Matrix metalloproteinase production by cultured human endometrial stromal cells: identification of interstitial collagenase, gelatinase-A, gelatinase-B, and stromelysin-1 and their differential regulation by interleukin-1 alpha and tumor necrosis factor-alpha. J Clin Endocrinol Metab. 1994;79(2): 530–536.
Terranova PF. Potential roles of tumor necrosis factor-alpha in follicular development, ovulation, and the life span of the corpus luteum. Domest Anim Endocrinol. 1997;14(1):1–15.
Marcinkiewicz JL, Krishna A, Cheung CM, Terranova PF. Oocytic tumor necrosis factor alpha: localization in the neonatal ovary and throughout follicular development in the adult rat. Biol Reprod. 1994;50(6):1251–1260.
Sancho-Tello M, Tash JS, Roby KF, Terranova PF. Effects of lipopolysaccharide on ovarian function in the pregnant mare serum gonadotropin-treated immature rat. Endocrine. 1993;1:503–511.
Kondo H, Maruo T, Mochizuki M. Immunohistochemical evidence for the presence of tumor necrosis factor-alpha in the infant and adult human ovary. Endocr J. 1995;42(6):771–780.
Li S, Maruo T, Ladines-Llave CA, Samoto T, Kondo H, Mochizuki M. Expression of transforming growth factor-alpha in the human ovary during follicular growth, regression and atresia. Endocr J. 1994;41(6):693–701.
Grazul-Bilska AT, Redmer DA, Bilski JJ, Jablonka-Shariff A, Doraiswamy V, Reynolds LP. Gap junctional proteins, connexin 26, 32, and 43 in sheep ovaries throughout the estrous cycle. Endocrine. 1998;8(3):269–279.
Lenhart JA, Downey BR, Bagnell CA. Connexin 43 gap junction protein expression during follicular development in the porcine ovary. Biol Reprod. 1998;58(2):583–590.
Melton CM, Zaunbrecher GM, Yoshizaki G, et al. Expression of connexin 43 mRNA and protein in developing follicles of prepubertal porcine ovaries. Comp Biochem Physiol B Biochem Mol Biol. 2001;130(1):43–55.
Li JR, Shen T, Wang YL, Wei QW, Shi FX. Expression of matrix metalloproteinases and ovarian morphological changes in androgenized cyclic female guinea pigs. Tissue Cell. 2016; 48(1):72–80.
Huet C, Monget P, Pisselet C, Hennequet C, Locatelli A, Monniaux D. Chronology of events accompanying follicular atresia in hypophysectomized ewes. Changes in levels of steroidogenic enzymes, connexin 43, insulin-like growth factor II/mannose 6 phosphate receptor, extracellular matrix components, and matrix metalloproteinases. Biol Reprod. 1998;58(1):175–185.
Scarano WR, Toledo FC, Guerra MT, et al. Long-term effects of developmental exposure to di-n-butyl-phthalate (DBP) on rat prostate: proliferative and inflammatory disorders and a possible role of androgens. Toxicology. 2009;262(3):215–223.
Gaide Chevronnay HP, Selvais C, Emonard H, Galant C, Marbaix E, Henriet P. Regulation of matrix metalloproteinases activity studied in human endometrium as a paradigm of cyclic tissue breakdown and regeneration. Biochim Biophys Acta. 2012; 1824(1):146–156.
Veiga-Lopez A, Astapova OI, Aizenberg EF, Lee JS, Padmanabhan V. Developmental programming: contribution of prenatal androgen and estrogen to estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biol Reprod. 2009;80(4): 718–725.
Curry TE Jr, Song L, Wheeler SE. Cellular localization of gelatinases and tissue inhibitors of metalloproteinases during follicular growth, ovulation, and early luteal formation in the rat. Biol Reprod. 2001;65(3):855–865.
Liao X, Thrasher JB, Pelling J, Holzbeierlein J, Sang QX, Li B. Androgen stimulates matrix metalloproteinase-2 expression in human prostate cancer. Endocrinology. 2003;144(5):1656–1663.
Thathapudi S, Kodati V, Erukkambattu J, Katragadda A, Addepally U, Hasan Q. Tumor necrosis factor-alpha and polycystic ovarian syndrome: a clinical, biochemical, and molecular genetic study. Genet Test Mol Biomarkers. 2014;18(9):605–609.
Gruss HJ. Molecular, structural, and biological characteristics of the tumor necrosis factor ligand superfamily. Int J Clin Lab Res. 1996;26(3):143–159.
Adams J, Liu Z, Ren YA, et al. Enhanced inflammatory transcriptome in the granulosa cells of women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2016;101(9):3459–3468.
Schmidt J, Weijdegard B, Mikkelsen AL, Lindenberg S, Nilsson L, Brannstrom M. Differential expression of inflammation-related genes in the ovarian stroma and granulosa cells of PCOS women. Mol Hum Reprod. 2014;20(1):49–58.
Caselli C. Role of adiponectin system in insulin resistance. Mol Genet Metab. 2014;113(3):155–160.
Durlej M, Knapczyk-Stwora K, Duda M, et al. Prenatal and neonatal exposure to the antiandrogen flutamide alters connexin 43 gene expression in adult porcine ovary. Domest Anim Endocrinol. 2011;40(1):19–29.
Wu CH, Yang JG, Yang JJ, et al. Androgen excess downregulates connexin43 in a human granulosa cell line. Fertil Steril. 2010;94(7):2938–2941.
Yang M, Li J, An Y, Zhang S. Effects of androgen on immunohistochemical localization of androgen receptor and connexin 43 in mouse ovary. Tissue Cell. 2015;47(5):526–532.
Shalev E, Goldman S, Ben-Shlomo I. The balance between MMP-9 and MMP-2 and their tissue inhibitor (TIMP)-1 in luteinized granulosa cells: comparison between women with PCOS and normal ovulatory women. Mol Hum Reprod. 2001;7(4):325–331.
Wu H, Yu K, Yang Z. Associations between TNF-alpha and interleukin gene polymorphisms with polycystic ovary syndrome risk: a systematic review and meta-analysis. J Assist Reprod Genet. 2015;32(4):625–634.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puttabyatappa, M., Irwin, A., Martin, J.D. et al. Developmental Programming: Gestational Exposure to Excess Testosterone Alters Expression of Ovarian Matrix Metalloproteases and Their Target Proteins. Reprod. Sci. 25, 882–892 (2018). https://doi.org/10.1177/1933719117697127
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117697127