Abstract
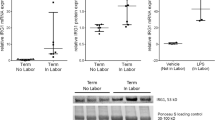
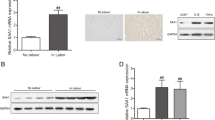
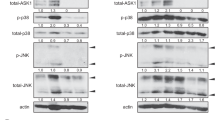
Preventing spontaneous preterm birth is one of the most important issues facing perinatal medicine today. The pathophysiology of preterm labor, the single biggest cause of preterm birth, is poorly understood. Inflammation, however, plays a significant role in the terminal processes of human labor, which include myometrial contractions. Nuclear factor κB (NF-κB) drives the transcription of proinflammatory mediators involved in the terminal effector pathways of human labor and delivery. Recent studies in nongestational tissues have shown that the adaptor protein p62 interacts with NF-κB to induce inflammation. The aim of this study was to determine the role of p62 in the genesis of NF-κB-induced proinflammatory and prolabur mediators. Human spontaneous term labor was associated with increased p62 messenger RNA (mRNA) and protein expression in myometrium. Myometrial cells treated with proinflammatory cytokines interleukin Iβ (IL-Iβ) and tumor necrosis factor alpha (TNF-α) also significantly increased p62 mRNA and protein expression. Functional studies using p62 small interfering RNA (siRNA) demonstrated a significant attenuation of TNF-α- and IL-Iβ-induced proinflammatory cytokines (IL-6) and chemokine (IL-8 and monocyte chemoattractant protein I [MCP-I]) mRNA expression and secretion, expression of cyclooxygenase 2, release of prostaglandin F2α (PGF2α), and expression of the prostaglandin F receptor (FP). In addition, siRNA knockdown of p62 significantly suppressed IL-Iβ- and TNF-α-induced NF-κB activation. Collectively, these studies suggest that p62 is involved in the genesis of NF-κB-induced proinflammatory and prolabor mediators.
Similar content being viewed by others
References
Kinney MV, Lawn JE, Howson CP, Belizan J. 15 million preterm births annually: what has changed this year? Reprod Health. 2012;9:28.
Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161.
Norman JE, Shennan AH. Prevention of preterm birth-why can’t we do any better? Lancet. 2012;381(9862):184–185.
Howson CP, Kinney MV, Lawn JE. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization; 2012.
Howson CP, Kinney MV, McDougall L, Lawn JE; Born Too Soon Preterm Birth Action Group. Born Toon Soon: preterm birth matters. Reprod Health. 2013;10(suppl 1):S1.
Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765.
Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–429.
Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9(1):41–45.
Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–236.
Rauk PN, Chiao JP. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol. 2000;43(3):152–159.
Bartlett SR, Sawdy R, Mann GE. Induction of cyclooxygenase-2 expression in human myometrial smooth muscle cells by interleukin-1beta: involvement of p38 mitogen-activated protein kinase. J Physiol. 1999;520(pt 2):399–406.
Erkinheimo TL, Saukkonen K, Narko K, Jalkanen J, Ylikorkala O, Ristimaki A. Expression of cyclooxygenase-2 and prostanoid receptors by human myometrium. J Clin Endocrinol Metab. 2000;85(9):3468–3475.
Bokstrom H, Brannstrom M, Alexandersson M, Norstrom A. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Hum Reprod. 1997;12(3):586–590.
Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66(2):445–449.
Lappas M, Rice GE. The role and regulation of the nuclear factor kappa B signalling pathway in human labour. Placenta. 2007;28(5-6):543–556.
Lappas M, Rice GE. Transcriptional regulation of the processes of human labour and delivery. Placenta. 2009;30(suppl A):S90–S95.
Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction. 2005;130(5):569–581.
Liong S, Lappas M. The stress-responsive heme oxygenase (HO)-1 isoenzyme is increased in labouring myometrium where it regulates contraction-associated proteins. Am J Reprod Immunol. 2015;74(1):62–76.
Khanjani S, Terzidou V, Johnson MR, Bennett PR. NFkappaB and AP-1 drive human myometrial IL8 expression. Mediators Inflamm. 2012;2012:504952.
Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137(6):1001–1004.
Duran A, Serrano M, Leitges M, et al. The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell. 2004;6(2):303–309.
Sanz L, Sanchez P, Lallena MJ, Diaz-Meco MT, Moscat J. The interaction of p62 with RIP links the atypical PKCs to NF-kappaB activation. EMBO J. 1999;18(11):3044–3053.
Duran A, Linares JF, Galvez AS, et al. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13(4):343–354.
Wooten MW, Geetha T, Seibenhener ML, Babu JR, Diaz-Meco MT, Moscat J. The p62 scaffold regulates nerve growth factor-induced NF-kappaB activation by influencing TRAF6 polyubiquitination. J Biol Chem. 2005;280(42):35625–35629.
Sanz L, Diaz-Meco MT, Nakano H, Moscat J. The atypical PKC-interacting protein p62 channels NF-kappaB activation by the IL-1-TRAF6 pathway. EMBO J. 2000;19(7):1576–1586.
Seibold K, Ehrenschwender M. p62 regulates CD40-mediated NFkappaB activation in macrophages through interaction with TRAF6. Biochem Biophys Res Commun. 2015;464(1):330–335.
Lee HM, Shin DM, Yuk JM, et al. Autophagy negatively regulates keratinocyte inflammatory responses via scaffolding protein p62/SQSTM1. J Immunol. 2011;186(2):1248–1558.
Mathew R, Karp CM, Beaudoin B, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137(6):1062–1075.
Lim R, Tran HT, Liong S, Barker G, Lappas M. The transcription factor interferon regulatory factor-1 (IRF1) plays a key role in the terminal effector pathways of human preterm labor. Biol Reprod. 2016;94(2):32.
Lim R, Barker G, Lappas M. A novel role for FOXO3 in human labor: increased expression in laboring myometrium, and regulation of proinflammatory and prolabor mediators in pregnant human myometrial cells. Biol Reprod. 2013;88(6):156.
Lim R, Barker G, Lappas M. TREM-1 expression is increased in human placentas from severe early-onset preeclamptic pregnancies where it may be involved in syncytialization. Reprod Sci. 2014;21(5):562–572.
Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23(4):257–273.
Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79(1):50–57.
Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol. 2010;88(4):625–633.
Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition — a review. Placenta. 2003;24(suppl A):S33–S46.
Lappas M, Rice GE. Phospholipase A2 isozymes in pregnancy and parturition. Prostaglandins Leukot Essent Fatty Acids. 2004;70(2):87–100.
Roh CR, Oh WJ, Yoon BK, Lee JH. Up-regulation of matrix metalloproteinase-9 in human myometrium during labour: a cytokine-mediated process in uterine smooth muscle cells. Mol Hum Reprod. 2000;6(1):96–102.
Tattersall M, Engineer N, Khanjani S, et al. Pro-labour myometrial gene expression: are preterm labour and term labour the same? Reproduction. 2008;135(4):569–579.
Tribe RM, Moriarty P, Dalrymple A, Hassoni AA, Poston L. Interleukin-1beta induces calcium transients and enhances basal and store operated calcium entry in human myometrial smooth muscle. Biol Reprod. 2003;68(5):1842–1849.
Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165(4 pt 1):969–971.
Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195(6):1578–1589.
Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167(4 pt 1):1041–1045.
Manley S, Williams JA, Ding WX. Role of p62/SQSTM1 in liver physiology and pathogenesis. Exp Biol Med (Maywood). 2013;238(5):525–538.
Seibenhener ML, Geetha T, Wooten MW. Sequestosome 1/p62 — more than just a scaffold. FEBS Lett. 2007;581(2):175–179.
Lim R, Barker G, Lappas M. The transcription factor Nrf2 is decreased after spontaneous term labour in human fetal membranes where it exerts anti-inflammatory properties. Placenta. 2015;36(1):7–17.
Lappas M, Yee K, Permezel M, Rice GE. Lipopolysaccharide and TNF-alpha activate the nuclear factor kappa B pathway in the human placental JEG-3 cells. Placenta. 2006;27(6-7):568–575.
Lappas M, Yee K, Permezel M, Rice GE. Sulfasalazine and BAY 11-7082 interfere with the nuclear factor-kappa B and I kappa B kinase pathway to regulate the release of proinflammatory cytokines from human adipose tissue and skeletal muscle in vitro. Endocrinology. 2005;146(3):1491–1497.
De Silva D, Mitchell MD, Keelan JA. Inhibition of choriodecidual cytokine production and inflammatory gene expression by selective I-kappaB kinase (IKK) inhibitors. Br J Pharmacol. 2010;160(7):1808–1822.
Keelan JA, Khan S, Yosaatmadja F, Mitchell MA. Prevention of inflammatory activation of human gestational membranes in an ex vivo model using a pharmacological NF-kappa B inhibitor. J Immunol. 2009;183(8):5270–5278.
Teramachi J, Silbermann R, Yang P, et al. Blocking the ZZ domain of sequestosome 1/p62 suppresses myeloma growth and osteoclast formation in vitro and induces dramatic bone formation in myeloma-bearing bones in vivo. Leukemia. 2016;30(2):390–398.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lappas, M. The Adaptor Protein p62 Mediates Nuclear Factor κB Activation in Response to Inflammation and Facilitates the Formation of Prolabor Mediators in Human Myometrium. Reprod. Sci. 24, 762–772 (2017). https://doi.org/10.1177/1933719116669058
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719116669058