Abstract
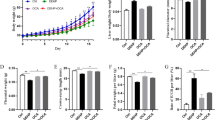
Intrahepatic cholestasis of pregnancy (ICP) is a disorder of bile acid (BA) synthesis, excretion, and metabolism, with systemic accumulation of BAs, which can lead to prematurity, fetal distress, and intrauterine death. Here, we investigate the expression of peroxisome proliferator-activated receptor alpha and cytochrome P450 oxysterol 7alpha-hydroxylase by exposing to 17α-ethynylestradiol with or without the estrogen receptor signaling pathway in pregnant rats with intrahepatic cholestasis. In vivo and in vitro evidences showed that estrogen receptor alpha (ERα) may be the key point of occurrence and development of intra-hepatic cholestasis in pregnant rats. Besides, the abnormalities in genes could be reversed by ERα small interfering RNA. Our findings provide the ERα-centered hypothesis on the mechanisms of ICP. New perspectives are emerging for the treatment of estrogen-induced hepatic complication.
Similar content being viewed by others
References
Diken Z, Usta IM, Nassar AH. A clinical approach to intrahepatic cholestasis of pregnancy. Am J Perinatol. 2014;31(1):1–8.
Leslie KK, Reznikov L, Simon FR, Fennessey PV, Reyes H, Ribalta J. Estrogens in intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2000;95(3):372–376.
Kaempffer AM, Vargas R, Sepúlveda L, Quintana A, Nocera C, Cumsille MA. Intrahepatic cholestasis in pregnancy and perinatal risk. Rev Chil Obstet Ginecol. 1990;55(1):41–45.
Rook M, Vargas J, Caughey A, Bacchetti P, Rosenthal P, Bull L. Fetal outcomes in pregnancies complicated by intrahepatic cho-lestasis of pregnancy in a Northern California cohort. PLoS One. 2012;7(3):e28343.
Shemer EW, Marschall HU, Ludvigsson JF, Stephansson O. Intra-hepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG. 2013;120(6):717–723.
Kohari KS, Ferrara LA, Carroll R, Capogna S, Ditchik A, Fox NS, Outcome after implementation of a new management strategy for intrahepatic cholestasis of pregnancy [published online August 8, 2016]. J Matern Fetal Neonatal Med. 2016.
Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897–906.
Rodriguez-Garay EA. Cholestasis: human disease and experimental animal models. Ann Hepatol. 2002;2(4):150–158.
Invernizzi P, Alvaro D, Crosignani A, Gaudio E, Podda M. Tamoxifen in treatment of primary biliary cirrhosis. Hepatology. 2004;39(4):1175–1176.
Reddy A, Prince M, James OF, Jain S, Bassendine MF. Tamoxifen: a novel treatment for primary biliary cirrhosis? Liver Int. 2004;24(3):194–197.
Martin KO, Reiss AB, Lathe R, Javitt NB. 7 alpha-hydroxylation of 27-hydroxycholesterol: biologic role in the regulation of cholesterol synthesis. J Lipid Res. 1997;38(5):1053–1058.
Diczfalusy U, Olofsson KE, Carlsson AM, et al. Marked upregu-lation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J Lipid Res. 2009;50(11):2258–2264.
Yantsevich AV, Dichenko YV, Mackenzie F, et al. Human steroid and oxysterol 7α-hydroxylase CYP7B1: substrate specificity, azole binding and misfolding of clinically relevant mutants. FEBS J. 2014;281(6):1700–1713.
Tang W, Pettersson H, Norlin M. Involvement of the PI3K/Akt pathway in estrogen-mediated regulation of human CYP7B1: identification of CYP7B1 as a novel target for PI3K/Akt and MAPK signalling. J Steroid Biochem Mol Biol. 2008;112(1-3): 63–73.
Dusell CD, Michihisa U, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22(1):65–77.
Lee WR, Ishikawa T, Umetani M. The interaction between metabolism, cancer an cardiovascular disease, connected be 27-hydroxycholesterol. Clin Lipidol. 2014;9(6):617–624.
Nicolas L, Sylvain P, Walter W. Sumoylated PPARalpha mediates sex-specific gene repression and protects the liver from estrogen-induced toxicity in mice. J Clin Invest. 2009;119(10):3138–3148.
Yoon M. The role of PPARα in lipid metabolism and obesity: focusing on the effects of estrogen on PPARα actions. Pharmacol Res. 2009;60(3):151–159.
Keller H, Givel F, Perroud M, Wahli W. Signaling cross-talk between peroxisome proliferator-activated receptor/retinoid X receptor and estrogen receptor through estrogen response elements. Mol Endocrinol. 1995;9(7):794–804.
Nunẽz SB, Medin JA, Braissant O, et al. Retinoid X receptor and peroxisome proliferator-activated receptor activate an estrogen responsive gene independent of the estrogen receptor. Mol Cell Endocrinol. 1997;127(1):27–40.
Wang X, Kilgore MW. Signal cross-talk between estrogen receptor alpha and beta and the peroxisome proliferator-activated receptor gamma1 in MDA-MB-231 and MCF-7 breast cancer cells. Mol Cell Endocrinol. 2002;194(1-2):123–133.
Tcherepanova I, Puigserver P, Norris JD, Spiegelman BM, McDonnell DP. Modulation of estrogen receptor-a transcriptional activity by the coactivator PGC-1. J Biol Chem. 2000;275(21):16302–16308.
Papacleovoulou G, Abu-Hayyeh S, Williamson C. Nuclear receptor-driven alterations in bile acid and lipid metabolic pathways during gestation. Biochim Biophys Acta. 2010;1812(8):879–887.
Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83.
Ribaux P, Gjinovci A, Sadowski HB, Iynedjian PB. Discrimination between signaling pathways in regulation of specific gene expression by insulin and growth hormone in hepatocytes. Endocrinology. 2002;143(10):3766–3772.
Carbonaro V, Caraci F, Giuffrida ML, et al. Enhanced expression of ERa in astrocytes modifies the response of cortical neurons to b-amyloid toxicity. Neurobiol Dis. 2009;33(3):415–421.
Rossi DV, Dai Y, Thomas P, et al. Estradiol-induced desensitization of 5-HT 1A receptor signaling in the paraventricular nucleus of the hypothalamus is independent of estrogen receptor-beta. Psychoneuroendocrinology. 2010;35(7):1023–1033.
Yamamoto Y, Moore R, Hess HA, et al. Estrogen receptor a mediates 17α-ethynylestradiol causing hepatotoxicity. J Biol Chem. 2006;281(24):16625–16631.
Ghonem NS, Assis DN, Boyer JL. Fibrates and cholestasis. Hepatology. 2015;62(2):635–643.
Chan AWH, Quaglia A, Haugk B, Burt A. Obstetric Liver Disease. Springer New York, NY: Atlas of Liver Pathology; 2014:221–224.
Chen A, Reyes A, Akeson R. A homopurine: homopyrimidine sequence derived from the rat neuronal cell adhesion molecule-encoding gene alters expression in transient transfections. Gene. 1993;128(2):211–218.
Jacquemin E, Cresteil D, Manouvrier S, Boute O, Hadchouel M. Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet. 1999;353(9148):210–211.
Meyers M, Slikker W, Pascoe G, Vore M. Characterization of cholestasis induced by estradiol-17beta-D-glucuronide in the rat. J Pharmacol Exp Ther. 1980;214(1):87–93.
Sano N, Takikawa H, Yamanaka M. Estradiol-17β-glucuronide-induced cholestasis: effects of ursodeoxycholate-3-O-glucuronide and 3, 7-disulfate. J Hepatol. 1993;17(2):241–246.
Guo RX, Wei LH, Tu Z, et al. 17 beta-estradiol activates PI3K/ Akt signaling pathway by estrogen receptor (ER)-dependent and ER-independent mechanisms in endometrial cancer cells. J Steroid Biochem Mol Biol. 2006;99(1):9–18.
Marino M, Pallottini V, Trentalance A. Estrogens cause rapid activation of IP3 -PKC-alpha signal transduction pathway in HEPG2 cells. Biochem Biophys Res Commun. 1998;245(1):254–258.
Barosso IR, Zucchetti AE, Boaglio AC, et al. Sequential activation of classic PKC and estrogen receptor a is involved in estradiol 17b-D-glucuronide-induced cholestasis. Plos One. 2012; 7(11):e50711
Bartella V, Rizza P, Barone I, et al. Estrogen receptor beta binds Sp1 and recruits a corepressor complex to the estrogen receptor alpha gene promoter. Breast Cancer Res Treat. 2012;134(2):569–581.
Nina H, Ashley P, Sandra A, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931.
Eyster KM. The estrogen receptors: an overview from different perspectives. Methods Mol Biol. 2016;1366:1-10.
Chu R, van Hasselt A, Vlantis AC, et al. The cross-talk between estrogen receptor and peroxisome proliferator-activated receptor gamma in thyroid cancer. Cancer. 2014;120(1):142–153.
Fruchart JC. Peroxisome proliferator-activated receptor-a activation and high-density lipoprotein metabolism. Am J Cardiol. 2001;88(12A):24–29.
Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/ specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol. 2008;41(5):263–275.
Yoon M. PPARa in Obesity: sex difference and estrogen involvement. PPAR Res. 2010;2010:pii: 584296.
Tang W, Eggertsen G, Chiang JY, Norlin M. Estrogen-mediated regulation of CYP7B1: a possible role for controlling DHEA levels in human tissues. J Steroid Biochem Mol Biol. 2006; 100(1-3):42–51.
Pettersson H, Lundqvist J, Norlin M. Effects of CYP7B1-mediated catalysis on estrogen receptor activation. Biochim Bio-phys Acta. 2010;1801(9):1090–1097.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, Z., Shi, Q. The Interaction of PPARα and CYP7B1 with ERα, β Impacted the Occurrence and Development of Intrahepatic Cholestasis in Pregnant Rats. Reprod. Sci. 24, 627–634 (2017). https://doi.org/10.1177/1933719116667223
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719116667223