Abstract
Introduction
Primary human vaginal cells derived from women with severe pelvic organ prolapse (POP-HVCs) demonstrate altered cellular characteristics as compared to cells derived from asymptomatic women (control-HVCs). Using computer-controllable Flexcell stretch unit, we examined whether POP-HVCs react differently to mechanical loading as compared to control-HVCs by the expression of extracellular matrix (ECM) components, cell–ECM adhesion proteins, and ECM degrading and maturating enzymes.
Methods
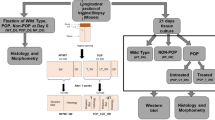
Vaginal tissue biopsies from premenopausal patients with Pelvic Organ Prolapse Quantification System stage ≥3 (n = 8) and asymptomatic controls (n = 7) were collected during vaginal hysterectomy or repair. Human vaginal cells were isolated by enzymatic digestion, seeded on collagen (COLI)-coated plates, and stretched (24 hours, 25% elongation). Total RNA was extracted, and 84 genes were screened using Human ECM and Adhesion Molecules polymerase chain reaction array; selected genes were verified by quantitative reverse transcription-polymerase chain reaction. Stretch-conditioned media (SCM) were collected and analyzed by protein array, immunoblotting, and zymography.
Results
In mechanically stretched control-HVCs, transcript levels of integrins (ITGA1, ITGA4, ITGAV, and ITGB1) and matrix metalloproteinases (MMPs) 2, 8, and 13 were downregulated (P < .05); in POP-HVCs, MMP1, MMP3, and MMP10, ADAMTS8 and 13, tissue inhibitor of metalloproteinases (TIMPs) 1 to 3, ITGA2, ITGA4, ITGA6, ITGB1, contactin (CNTN1), catenins (A1 and B1), and laminins (A3 and C1) were significantly upregulated, whereas COLs (1, 4, 5, 6, 11, and 12) and LOXL1 were downregulated. Human vaginal cells massively secrete MMPs and TIMPs proteins; MMP1, MMP8, MMP9 protein expression and MMP2 gelatinase activity were increased, whereas TIMP2 decreased in SCM from POP-HVCs compared to control-HVCs.
Conclusions
Primary human vaginal cells derived from women with severe pelvic organ prolapse and control-HVCs react differentially to in vitro mechanical stretch. Risk factors that induce stretch may alter ECM composition and cell–ECM interaction in pelvic floor tissue leading to the abatement of pelvic organ support and subsequent POP development.
Similar content being viewed by others
References
Delancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717–1724.
Moalli PA, Jones IS, Meyn LA, Zyczynski HM. Risk factors associated with pelvic floor disorders in women undergoing surgical repair. Obstet Gynecol. 2003;101(5 pt 1):869–874.
Whitcomb EL, Rortveit G, Brown JS, et al. Racial differences in pelvic organ prolapse. Obstet Gynecol. 2009;114(6):1271–1277.
Salvatore S, Siesto G, Serati M. Risk factors for recurrence of genital prolapse. Curr Opin Obstet Gynecol. 2010;22(5):420–424.
Smith FJ, Holman CD, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116(5):1096–1100.
Abramowitch SD, Feola A, Jallah Z, Moalli PA. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144(suppl 1):S146–S158.
Barber MD. Symptoms and outcome measures of pelvic organ prolapse. Clin Obstet Gynecol. 2005;48(3):648–661.
Ingber D. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3(5):841–848.
Martinez-Lemus LA, Wu X, Wilson E, et al. Integrins as unique receptors for vascular control. J Vasc Res. 2003;40(3):211–233.
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506.
Deprest J, Feola A. The need for preclinical research on pelvic floor reconstruction. BJOG. 2013;120(2):141–143.
Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Surgical treatment of pelvic organ prolapse: a historical review with emphasis on the anterior compartment. Int Urogynecol J. 2013;24(10):1593–1602.
Kufaishi H. Comparative characterization of vaginal cells derived from premenopausal women with and without severe pelvic organ prolapse. Reprod Sci. 2015.
Al-Taher H, Sutherst JR, Richmond DH, Brown MC. Vaginal pressure as an index of intra-abdominal pressure during urodynamic evaluation. Br J Urol. 1987;59(6):529–532.
O’Dell KK, Morse AN, Crawford SL, Howard A. Vaginal pressure during lifting, floor exercises, jogging, and use of hydraulic exercise machines. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(12):1481–1489.
Kasper G, Glaeser JD, Geissler S, et al. Matrix metalloprotease activity is an essential link between mechanical stimulus and mesenchymal stem cell behavior. Stem Cells. 2007;25(8):1985–1994.
Yang CM, Chien CS, Yao CC, Hsiao LD, Huang YC, Wu CB. Mechanical strain induces collagenase-3 (MMP13) expression in MC3T3-E1 osteoblastic cells. J Biol Chem. 2004;279(21):22158–22165.
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363.
Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146(1):56–66.
Alarab M, Bortolini MA, Drutz H, Lye S, Shynlova O. LOX family enzymes expression in vaginal tissue of premenopausal women with severe pelvic organ prolapse. Int Urogynecol J. 2010;21(11):1397–1404.
Alarab M, Kufaishi H, Lye S, Drutz H, Shynlova O. Expression of extracellular matrix-remodeling proteins is altered in vaginal tissue of premenopausal women with severe pelvic organ prolapse. Reprod Sci. 2014;21(6):704–715.
Shynlova O, Bortolini MA, Alarab M. Genes responsible for vaginal extracellular matrix metabolism are modulated by women’s reproductive cycle and menopause. Int Braz J Urol. 2013;39(2):257–267.
Medel S, Alarab M, Kufaishi H, Drutz H, Shynlova O. Attachment of primary vaginal fibroblasts to absorbable and nonabsorbable implant materials coated with platelet-rich plasma: potential application in pelvic organ prolapse surgery. Female Pelvic Med Reconstr Surg. 2015;21(4):190–197.
Bortolini MA, Shynlova O, Drutz HP, et al. Expression of bone morphogenetic protein-1 in vaginal tissue of women with severe pelvic organ prolapse. Am J Obstet Gynecol. 2011;204:544–548.
Moalli PA, Shand SH, Zyczynski HM, Gordy SC, Meyn LA. Remodeling of vaginal connective tissue in patients with prolapse. Obstet Gynecol. 2005;106(5 pt 1):953–963.
Mosier E, Lin VK, Zimmern P. Extracellular matrix expression of human prolapsed vaginal wall. Neurourol Urodyn. 2010;29(4):582–586.
Zhou L, Lee JH, Wen Y, et al. Biomechanical properties and associated collagen composition in vaginal tissue of women with pelvic organ prolapse. J Urol. 2012;188(3):875–880.
Berger MB, Ramanah R, Guire KE, Delancey JO. Is cervical elongation associated with pelvic organ prolapse?. Int Urogynecol J. 2012;23(8):1095–1103.
Phillips CH, Anthony F, Benyon C, Monga AK. Collagen metabolism in the uterosacral ligaments and vaginal skin of women with uterine prolapse. BJOG. 2006;113(1):39–46.
Liang CC, Huang HY, Tseng LH, Chang SD, Lo TS, Lee CL. Expression of matrix metalloproteinase-2 and tissue inhibitors of metalloproteinase-1 (TIMP1, TIMP2 and TIMP3) in women with uterine prolapse but without urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 2010;153(1):94–98.
Word RA, Pathi S, Schaffer JI. Pathophysiology of pelvic organ prolapse. Obstet Gynecol Clin North Am. 2009;36(3):521–539.
Ruiz-Zapata AM, Kerkhof MH, Zandieh-Doulabi B, Brolmann HA, Smit TH, Helder MN. Fibroblasts from women with pelvic organ prolapse show differential mechanoresponses depending on surface substrates. Int Urogynecol J. 2013;24(9):1567–1575.
Feola A, Abramowitch S, Jones K, Stein S, Moalli P. Parity negatively impacts vaginal mechanical properties and collagen structure in rhesus macaques. Am J Obstet Gynecol. 2010;203(6):595–598.
Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA. Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Am J Obstet Gynecol. 2008;198(5):590–596.
Yurchenco PD, Wadsworth WG. Assembly and tissue functions of early embryonic laminins and netrins. Curr Opin Cell Biol. 2004;16(5):572–579.
Zong W, Jallah ZC, Stein SE, Abramowitch SD, Moalli PA. Repetitive mechanical stretch increases extracellular collagenase activity in vaginal fibroblasts. Female Pelvic Med Reconstr Surg. 2010;16(5):257–262.
Hawwa RL, Hokenson MA, Wang Y, Huang Z, Sharma S, Sanchez-Esteban J. Differential expression of MMP2 and -9 and their inhibitors in fetal lung cells exposed to mechanical stretch: regulation by IL-10. Lung. 2011;189(4):341–349.
Zhou D, Lee HS, Villarreal F, et al. Differential MMP2 activity of ligament cells under mechanical stretch injury: an in vitro study on human ACL and MCL fibroblasts. J Orthop Res. 2005;23(4):949–957.
Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839.
Lelievre E, Hinek A, Lupu F, Buquet C, Soncin F, Mattot V. VE-statin/egfl7 regulates vascular elastogenesis by interacting with lysyl oxidases. EMBO J. 2008;27(12):1658–1670.
Kerkhof MH, Ruiz-Zapata AM, Bril H, et al. Changes in tissue composition of the vaginal wall of premenopausal women with prolapse. Am J Obstet Gynecol. 2014;210(2):168–169.
Ruiz-Zapata AM, Kerkhof MH, Zandieh-Doulabi B, Brolmann HA, Smit TH, Helder MN. Functional characteristics of vaginal fibroblastic cells from premenopausal women with pelvic organ prolapse. Mol Hum Reprod. 2014;20(11):1135–1143.
Gao P, Jiang D, Liu W, Li H, Li Z. Urine-derived stem cells, a new source of seed cells for tissue engineering [published online February 20, 2015]. Curr Stem Cell Res Ther. 2015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kufaishi, H., Alarab, M., Drutz, H. et al. Static Mechanical Loading Influences the Expression of Extracellular Matrix and Cell Adhesion Proteins in Vaginal Cells Derived From Premenopausal Women With Severe Pelvic Organ Prolapse. Reprod. Sci. 23, 978–992 (2016). https://doi.org/10.1177/1933719115625844
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719115625844