Abstract
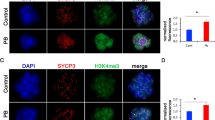
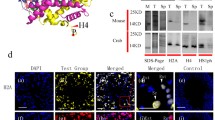
Histone acetylation has been known to be significant in spermatogenesis. Histone acetylation is regulated by the act of histone deacetylases (HDACs) and histone acetyltransferases (HATs). We investigated the link between expression levels of HDACs and HATs in mouse sperm and subsequent blastocyst formation rate. In the univariate analysis, expression levels of HDAC1 and HAT were generally not associated with the blastocyst formation rate. When divided by the mature oocyte number category, a significant positive association was observed between the expression levels of HDAC1 and the blastocyst-forming rate in the highest (> 75th) percentile group (a group with ≥34 mature oocytes). In conclusion, expression of sperm HDAC1 could be considered as a possible predictor of embryo development in mice with high ovarian response.
Similar content being viewed by others
References
Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–599.
Turner BM. Histone acetylation and an epigenetic code. Bioes-says. 2000;22(9):836–845.
Davie JR. Covalent modifications of histones: expression from chromatin templates. Curr Opin Genet Dev. 1998;8(2): 173–178.
Meistrich ML, Trostle-Weige PK, Lin R, Bhatnagar YM, Allis CD. Highly acetylated H4 is associated with histone displacement in rat spermatids. Mol Reprod Dev. 1992;31(3):170–181.
Hazzouri M, Pivot-Pajot C, Faure AK, et al. Regulated hyperacetylation of core histones during mouse spermatogenesis: involvement of histone deacetylases. Eur J Cell Biol. 2000;79(12): 950–960.
Kim JH, Jee BC, Lee JM, Suh CS, Kim SH. Histone acetylation level and histone acetyltransferase/deacetylase activity in ejaculated sperm from normozoospermic men. Yonsei Med J. 2014; 55(5):1333–1340.
Steger K, Cavalcanti MC, Schuppe HC. Prognostic markers for competent human spermatozoa: fertilizing capacity and contribution to the embryo. Int J Androl. 2011;34(6 pt 1):513–527.
Zhang H, Xiao Y, Wang X, et al. Effects of histone deacetylase inhibitors on the early development of bovine androgenetic embryos. Cell Reprogram. 2014;16(1):54–64.
Oliveira CS, Saraiva NZ, de Souza MM, Tetzner TA, de Lima MR, Garcia JM. Effects of histone hyperacetylation on the preim-plantation development of male and female bovine embryos. Reprod Fertil Dev. 2010;22(6):1041–1048.
Simon L, Murphy K, Shamsi MB, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014;29(11):2402–2412.
McReynolds S, Dzieciatkowska M, Stevens J, Hansen KC, Schoolcraft WB, Katz-Jaffe MG. Toward the identification of a subset of unexplained infertility: a sperm proteomic approach. Fertil Steril. 2014;102(3):692–699.
Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;403(6769):501–502.
Lalancette C, Miller D, Li Y, Krawetz SA. Paternal contributions: new functional insights for spermatozoal RNA. J Cell Biochem. 2008;104(5):1570–1579.
Jin J, Huang X, Pan C, et al. Effect of sperm DNA fragmentation on IVF/ICSI clinical outcomes is diversified in women with different ovarian reserve. Abstract presented at 2014 Annual meeting of American Society of Reproductive Medicine.
Kistler WS, Henriksen K, Mali P, Parvinen M. Sequential expression of nucleoproteins during rat spermiogenesis. Exp Cell Res. 1996;225(2):374–381.
Sonnack V, Failing K, Bergmann M, Steger K. Expression of hyperacetylated histone H4 during normal and impaired human spermatogenesis. Andrologia. 2002;34(6):384–390.
Grozinger CM, Schreiber SL. Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem Biol. 2002;9(1):3–16.
Garrido N, Meseguer M, Alvarez J, Simón C, Pellicer A, Remohí J. Relationship among standard semen parameters, glutathione peroxidase/glutathione reductase activity, and mRNA expression and reduced glutathione content in ejaculated spermatozoa from fertile and infertile men. Fertil Steril. 2004;82(suppl 3): 1059–1066.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J., Kim, JH., Jee, BC. et al. Is There a Link Between Expression Levels of Histone Deacetylase/Acetyltransferase in Mouse Sperm and Subsequent Blastocyst Development?. Reprod. Sci. 22, 1387–1392 (2015). https://doi.org/10.1177/1933719115580997
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719115580997