Abstract
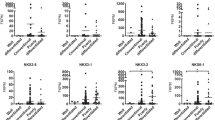
Uterine leiomyosarcoma is a relatively rare malignancy with high mortality due to metastasis and chemoresistance. Leiomyosarcomas share similar morphological characteristics with leiomyomas which are considered to have the potential of transformation into leiomyosarcoma. Accumulated evidence suggests that microRNAs acting as regulators of gene expression at the posttranscriptional level play key roles in diverse biological processes including cellular transformation and tumorigenesis. We hypothesized that miR-200c, whose expression is altered in leiomyomas, equally plays a key role in pathogenesis of leiomyosarcoma. Using SK-LMS-1 leiomyosarcoma cell line as an in vitro model here, we found that the level of expression of miR-200c was significantly lower as compared to isolated leiomyoma smooth muscle cells. Overexpression (gain-of-function) of miR-200c in SK-LMS-1 through direct interaction with 3′-untranslated region of IKBKB, IL8, CDK2, and CCNE2, respectively, resulted in suppression of their expression as determined by quantitative polymerase chain reaction and Western blot analysis. Additionally, gain-of-function of miR-200c through inhibition of IKBKB expression resulted in decreased p65 transcriptional activity in IL8 promoter. Gain-of-function of miR-200c also increased SK-LMS-1 caspase 3/7 activity and inhibited their proliferation and migration. In summary, the results suggest that a progressive decline in miR-200c expression which alters transcriptional regulation of specific target genes that control nuclear factor-κB signaling pathway, inflammation, cell cycle, and migration, in part may promote development and progression of leiomyosarcomas, including their transformation from leiomyomas.
Similar content being viewed by others
References
Shah SH, Jagannathan JP, Krajewski K, O’Regan KN, George S, Ramaiya NH. Uterine sarcomas: then and now. AJR. 2012;199(1): 213–223.
Kobayashi H, Uekuri C, Akasaka J, et al. The biology of uterine sarcomas: a review and update. Mol Clin Oncol. 2013;1(4):599–609.
Segars JH, Parrott EC, Nagel JD, et al. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Hum Reprod Update. 2014;20(3):309–333.
Raspollini MR, Amunni G, Villanucci A, et al. c-Kit expression in patients with uterine leiomyosarcomas: a potential alternative therapeutic treatment. Clin Cancer Res. 2004;10(10):3500–3503.
Kefeli M, Yildiz L, Kaya FC, Aydin O, Kandemir B. Fascin expression in uterine smooth muscle tumors. Int J Gynecol Pathol. 2009;28(4):328–333.
Koivisto-Korander R, Butzow R, Koivisto AM, Leminen A. Immunohistochemical studies on uterine carcinosarcoma, leiomyosarcoma, and endometrial stromal sarcoma: expression and prognostic importance of ten different markers. Tumour Biol. 2011;32(3):451–459.
Dhingra S, Rodriguez ME, Shen Q, et al. Constitutive activation with overexpression of the mTORC2-phospholipase D1 pathway in uterine leiomyosarcoma and STUMP: morphoproteomic analysis with therapeutic implications. Int J Clin Exp Pathol. 2011;4(2):134–146.
Hayashi T, Horiuchi A, Sano K, et al. Involvement of proteasome beta1i subunit, LMP2, on development of uterin leiomyosarcma. North Am J Med Sci. 2011;3(9):394–399.
Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12(2):99–110.
Medina PP, Slack FJ. microRNAs and cancer: an overview. Cell Cycle (Georgetown, Tex). 2008;7(16):2485–2492.
Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27(45):5959–5974.
Luo X, Chegini N. The expression and potential regulatory function of microRNAs in the pathogenesis of leiomyoma. Semin Reprod Med. 2008;26(6):500–514.
Subramanian S, Lui WO, Lee CH, et al. MicroRNA expression signature of human sarcomas. Oncogene. 2008;27(14): 2015–2026.
Kowalewska M, Bakula-Zalewska E, Chechlinska M, et al. microRNAs in uterine sarcomas and mixed epithelialmesenchymal uterine tumors: a preliminary report. Tumour Biol. 2013;34(4):2153–2160.
Guled M, Pazzaglia L, Borze I, et al. Differentiating soft tissue leiomyosarcoma and undifferentiated pleomorphic sarcoma: a miRNA analysis. Genes Chromosomes Cancer. 2014;53(8):693–702.
Danielson LS, Menendez S, Attolini CS, et al. A differentiation-based microRNA signature identifies leiomyosarcoma as a mesenchymal stem cell-related malignancy. Am J Pathol. 2010; 177(2):908–917.
Chuang TD, Panda H, Luo X, Chegini N. miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocr Relat Cancer. 2012;19(4):541–556.
Panda H, Pelakh L, Chuang TD, Luo X, Bukulmez O, Chegini N. Endometrial miR-200c is altered during transformation into cancerous states and targets the expression of ZEBs, VEGFA, FLT1, IKKbeta, KLF9, and FBLN5. Reprod Sci (Thousand Oaks, Calif). 2012;19(8):786–796.
Lee JW, Park YA, Choi JJ, et al. The expression of the miRNA-200 family in endometrial endometrioid carcinoma. Gynecol Oncol. 2011;120(1):56–62.
Chuang TD, Khorram O. miR-200c Regulates IL8 expression by targeting IKBKB: a potential mediator of inflammation in leiomyoma pathogenesis. PloS One. 2014;9(4):e95370.
Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141.
Chuang TD, Luo X, Panda H, Chegini N. miR-93/106b and their host gene, MCM7, are differentially expressed in leiomyomas and functionally target F3 and IL-8. Mol Endocrinol. 2012;26(6): 1028–1042.
Yu Z, Willmarth NE, Zhou J, et al. microRNA 17/20 inhibits cellular invasion and tumor metastasis in breast cancer by heterotypic signaling. Proc Natl Acad Sci U S A. 2010;107(18): 8231–8236.
Chen R, Alvero AB, Silasi DA, et al. Regulation of IKKbeta by miR-199a affects NF-kappaB activity in ovarian cancer cells. Oncogene. 2008;27(34):4712–4723.
Djuranovic S, Nahvi A, Green R. A parsimonious model for gene regulation by miRNAs. Science (New York, NY). 2011;331(6017): 550–553.
Prislei S, Martinelli E, Mariani M, et al. MiR-200c and HuR in ovarian cancer. BMC Cancer. 2013; 13:72.
Pecot CV, Rupaimoole R, Yang D, et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun. 2013;4:2427.
Wang S, Liu Z, Wang L, Zhang X. NF-kappaB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009; 6(5):327–334.
Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science (New York, NY). 1999;284(5412):309–313.
Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883.
Hu MC, Lee DF, Xia W, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004; 117(2):225–237.
Song L, Huang Q, Chen K, et al. miR-218 inhibits the invasive ability of glioma cells by direct downregulation of IKK-beta. Biochem Biophys Res Commun. 2010;402:135–140.
Dai L, Gu L, Di W. MiR-199a attenuates endometrial stromal cell invasiveness through suppression of the IKKbeta/NF-kappaB pathway and reduced interleukin-8 expression. Mol Hum Reprod. 2012;18(3):136–145.
Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27(42):5643–5647.
Bhattacharyya S, Balakathiresan NS, Dalgard C, et al. Elevated miR-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8. J Biol Chem. 2011;286(13): 11604–11615.
Musgrove EA. Cyclins: roles in mitogenic signaling and oncogenic transformation. Growth factors (Chur, Switzerland). 2006; 24(1):13–19.
Cai J, Liu X, Cheng J, et al. MicroRNA-200 is commonly repressed in conjunctival MALT lymphoma, and targets cyclin E2. Graefes Arch Clin Exp Ophthalmol. 2012;250(4):523–531.
Baek WK, Kim D, Jung N, et al. Increased expression of cyclin G1 in leiomyoma compared with normal myometrium. Am J Obstet Gynecol. 2003;188(3):634–639.
Shime H, Kariya M, Orii A, et al. Tranilast inhibits the proliferation of uterine leiomyoma cells in vitro through G1 arrest associated with the induction of p21(waf1) and p53. J Clin Endocrinol Metab. 2002;87(12):5610–5617.
Dobashi Y, Noguchi T, Nasuno S, Katayama K, Kameya T. CDK-inhibitors-associated kinase activity: a possible determinant of malignant potential in smooth muscle tumors of the external soft tissue. Int J Cancer. 2001;94(3):353–362.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chuang, TD., Ho, M. & Khorram, O. The Regulatory Function of miR-200c on Inflammatory and Cell-Cycle Associated Genes in SK-LMS-1, A Leiomyosarcoma Cell Line. Reprod. Sci. 22, 563–571 (2015). https://doi.org/10.1177/1933719114553450
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719114553450