Abstract
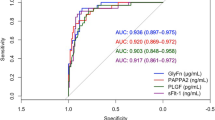
An overrepresentation of adverse pregnancy outcomes has been observed in pregnancies associated with a male fetus. We investigated the association between fetal gender and candidate biomarkers for preeclampsia. Proteins were quantified in samples taken at 20 weeks from women recruited to the SCreening fOr Pregnancy Endpoints (SCOPE) study (preeclampsia n = 150; no preeclampsia n = 450). In contrast to placental growth factor, soluble endoglin, and insulin-like growth factor acid labile subunit, levels of metallopeptidase domain 12 (ADAM12) at 20 weeks were dependent on fetal gender in pregnancies complicated by preeclampsia, for male (n = 73) fetuses the multiples of the median (MoM; interquartile range [IQR] 1.1–1.5) was 1.3, whereas for female fetuses (n = 75) MoM was 1.1 (1.0–1.3); P < .01. Prediction of preeclampsia using ADAM12 levels was improved for pregnancies associated with a male fetus (area under receiver–operator curve [AUC] 0.73 [95% confidence interval [CI] 0.67–0.80]) than that of a female fetus (AUC 0.62 [0.55–0.70]); P = .03. The data presented here fit a contemporary hypothesis that there is a difference between the genders in response to an adverse maternal environment and suggest that an alteration in ADAM12 may reflect an altered placental response in pregnancies subsequently complicated by preeclampsia.
Similar content being viewed by others
References
Byrne J, Warburton D. Male excess among anatomically normal fetuses in spontaneous abortions. Am J Med Genet. 1987;26(3):605–611.
James WH. Sex ratios of offspring and the causes of placental pathology. Human Reproduction. 1995;10(6):1403–1406.
Vatten LJ, Skjaerven R. Offspring sex and pregnancy outcome by length of gestation. Early Hum Dev. 2004;76(1):47–54.
Edwards A, Megens A, Peek M, Wallace EM. Sexual origins of placental dysfunction. Lancet. 2000;355(9199):203–204.
Walker MG, Fitzgerald B, Keating S, Ray JG, Windrim R, Kingdom JC. Sex-specific basis of severe placental dysfunction leading to extreme preterm delivery. Placenta. 2012;33(7):568–571.
Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci U S A. 2006;103(14):5478–5483.
Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31 suppl:S33–S39.
World Health Organisation. The World Health Report 2005-Make Every Mother and Child Count. Geneva: World Health Organisation; 2005.
Centre for Maternal and Child Enquiries (CMACE). Perinatal Mortality 2009: United Kingdom. London: CMACE;2011.
Bukowski R. Stillbirth and fetal growth restriction. Clin Obstet Gynecol. 2010;53(3):673–680.
Barker DJ. Fetal growth and adult disease. Br J Obstet Gynaecol. 1992;99(4):275–276.
North RA, McCowan LM, Dekker GA, et al. Clinical risk prediction for pre-eclampsia in nulliparous women: development of model in international prospective cohort. BMJ. 2011;342(1):d1875.
Kenny LC, Broadhurst DI, Dunn W, et al. Robust early pregnancy prediction of later preeclampsia using metabolomic biomarkers. Hypertension. 2010;56(4):741–749.
Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenat Diagn. 2011;31(1):66–74.
Myatt L, Clifton RG, Roberts JM, et al. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet Gynecol. 2012;119(6):1234–1242.
Myers JE, Kenny L, McCowan L, et al. Angiogenic factors combined with clinical risk factors to predict preterm pre-eclampsia in nulliparous women: apredictive test accuracy study. BJOG. 2013;120(10):1215–1223. doi: 10.1111/471-0528.12195.
Poon LC, Chelemen T, Granvillano O, Pandeva I, Nicolaides KH. First-trimester maternal serum a disintegrin and metalloprotease 12 (ADAM12) and adverse pregnancy outcome. Obstet Gynecol. 2008;112(5):1082–1090.
Laigaard J, Sorensen T, Placing S, et al. Reduction of the disintegrin and metalloprotease ADAM12 in preeclampsia. Obstet Gynecol. 2005;106(1):144–149.
Spencer K, Cowans NJ, Stamatopoulou A. ADAM12s in maternal serum as a potential marker of pre-eclampsia. Prenat Diagn. 2008;28(3):212–216.
Matwejew E, Cowans NJ, Stamatopoulou A, Spencer K, von Kaisenberg CS. Maternal serum ADAM-12 as a potential marker for different adverse pregnancy outcomes. Fetal Diagn Ther. 2010;27(1):32–39.
Myers JE, Tuytten R, Thomas G, et al. Integrated proteomics pipeline yields novel biomarkers for predicting preeclampsia. Hypertension. 2013;61(6):1281–1288.
Gentleman R, Hornik K, Leisch F. R 1.5 and the Bioconductor 1.0 releases. Comput Stat Data Analysis. 2002;39(4):557–558.
Gilpin BJ, Loechel F, Mattei MG, Engvall E, Albrechtsen R, Wewer UM. A novel, secreted form of human ADAM 12 (meltrin alpha) provokes myogenesis in vivo. J Biol Chem. 1998;273(1):157–166.
Laigaard J, Sorensen T, Frohlich C, et al. ADAM12: a novel first-trimester maternal serum marker for Down syndrome. Prenat Diagn. 2003;23(13):1086–1091.
Sahraravand M, Jarvela IY, Laitinen P, Tekay AH, Ryynanen M. The secretion of PAPP-A, ADAM12, and PP13 correlates with the size of the placenta for the first month of pregnancy. Placenta. 2011;32(12):999–1003.
Christians JK, Gruslin A. Altered levels of insulin-like growth factor binding protein proteases in preeclampsia and intrauterine growth restriction. Prenat Diagn. 2010;30(9):815–820.
Cowans NJ, Spencer K. First-trimester ADAM12 and PAPP-A as markers for intrauterine fetal growth restriction through their roles in the insulin-like growth factor system. Prenat Diagn. 2007;27(3):264–271.
Laigaard J, Larsen SO, Pedersen NG, et al. ADAM 12-S in first trimester: fetal gender, smoking and maternal age influence the maternal serum concentration. Prenat Diagn. 2009;29(5):525–527.
Bestwick JP, George LM, Wu T, Morris JK, Wald NJ. The value of early second trimester PAPP-A and ADAM12 in screening for pre-eclampsia. J Med Screen. 2012;19(1):51–54.
Geary MP, Pringle PJ, Rodeck CH, Kingdom JC, Hindmarsh PC. Sexual dimorphism in the growth hormone and insulin-like growth factor axis at birth. J Clin Endocrinol Metab. 2003;88(8):3708–3714.
Clifton VL, Hodyl NA, Murphy VE, Giles WB, Baxter RC, Smith R. Effect of maternal asthma, inhaled glucocorticoids and cigarette use during pregnancy on the newborn insulin-like growth factor axis. Growth Horm IGF Res. 2010;20(1):39–48.
Iniguez G, Argandona F, Medina P, et al. Acid-labile subunit (ALS) gene expression and protein content in human placentas: differences according to birth weight. J Clin Endocrinol Metab. 2011;96(1):187–191.
Baxter RC. Circulating levels and molecular distribution of the acid-labile (alpha) subunit of the high molecular weight insulinlike growth factor-binding protein complex. J Clin Endocrinol Metabol. 1990;70(5):1347–1353.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Myers, J.E., Thomas, G., Tuytten, R. et al. Mid-Trimester Maternal ADAM12 Levels Differ According to Fetal Gender in Pregnancies Complicated by Preeclampsia. Reprod. Sci. 22, 235–241 (2015). https://doi.org/10.1177/1933719114537713
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719114537713