Abstract
Preeclampsia (PE) remains a major cause of maternal/fetal morbidity–mortality worldwide. The first stage of PE is characterized by placental hypoxia due to a relative reduction in uteroplacental blood flow, resulting from restricted trophoblast invasion. However, hypoxia is also an essential element for the success of invasion. Under hypoxic conditions, 2-methoxyestradiol (2-ME) could induce the differentiation of cytotrophoblast cells into an invasive phenotype in culture. 2-Methoxyestradiol is generated by catechol-O-methyltransferase, an enzyme involved in the metabolic pathway of estrogens. During pregnancy, circulating 2-ME levels increase significantly when compared to the menstrual cycle. Interestingly, plasma levels of 2-ME are lower in women with PE than in controls, and these differences are apparent weeks or even months before the clinical manifestations of the disease. This article reviews the metabolic pathways involved in 2-ME synthesis and discusses the roles of these pathways in normal and abnormal pregnancies, with particular emphasis on PE.
Similar content being viewed by others
References
ACOG technical bulletin. Hypertension in pregnancy. Number 219—January 1996 (replaces no. 91, February 1986). Committee on technical bulletins of the American college of obstetricians and gynecologists. Int J Gynaecol Obstet. 1996;53(2):175–183.
Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974.
McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–930.
Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22.
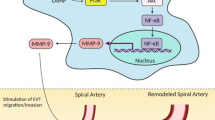
Knofler M, Pollheimer J. IFPA award in placentology lecture: molecular regulation of human trophoblast invasion. Placenta. 2012;33(suppl):S55–S62.
Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175(5):1365–1370.
Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11(5):309–316.
Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12(4):301–308.
Pijnenborg R, Vercruysse L, Brosens I. Deep placentation. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):273–285.
Hunkapiller NM, Fisher SJ. Chapter 12. Placental remodeling of the uterine vasculature. Methods Enzymol. 2008;445:281–302.
Whitley GS, Cartwright JE. Trophoblast-mediated spiral artery remodelling: a role for apoptosis. J Anat. 2009;215(1):21–26.
Lyall F. Mechanisms regulating cytotrophoblast invasion in normal pregnancy and pre-eclampsia. Aust N Z J Obstet Gynaecol. 2006;46(4):266–273.
Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191.
Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93(10):1049–1059.
Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80(2):283–285.
Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277(5332):1669–1672.
Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8(5):588–594.
Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270(3):1230–1237.
Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275.
Caniggia I, Mostachfi H, Winter J, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest. 2000; 105(5):577–587.
Librach CL, Werb Z, Fitzgerald ML, et al.. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113(2):437–449.
Lee SB, Wong AP, Kanasaki K, et al. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am J Pathol. 2010;176(2):710–720.
Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 pt 1):499–506.
Kanasaki K, Palmsten K, Sugimoto H, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453(7198):1117–1121.
LaVallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002;62(13):3691–3697.
Schumacher G, Kataoka M, Roth JA, Mukhopadhyay T. Potent antitumor activity of 2-methoxyestradiol in human pancreatic cancer cell lines. Clin Cancer Res. 1999;5(3):493–499.
Fotsis T, Zhang Y, Pepper MS, et al. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature. 1994;368(6468):237–239.
Zacharia LC, Jackson EK, Gillespie DG, Dubey RK. Increased 2-methoxyestradiol production in human coronary versus aortic vascular cells. Hypertension. 2001;37(2 part 2):658–662.
Shang W, Konidari I, Schomberg DW. 2-Methoxyestradiol, an endogenous estradiol metabolite, differentially inhibits granulosa and endothelial cell mitosis: a potential follicular antiangiogenic regulator. Biol Reprod. 2001;65(2):622–627.
Becker CM, Rohwer N, Funakoshi T, et al. 2-methoxyestradiol inhibits hypoxia-inducible factor-1 {alpha} and suppresses growth of lesions in a mouse model of endometriosis. Am J Pathol. 2008; 172(2):534–544.
Lakhani NJ, Sarkar MA, Venitz J, Figg WD. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy. 2003;23(2): 165–172.
Pasquier E, Sinnappan S, Munoz MA, Kavallaris M. ENMD-1198, a new analogue of 2-methoxyestradiol, displays both antiangiogenic and vascular-disrupting properties. Mol Cancer Ther. 2010;9(5):1408–1418.
Hou Y, Meyers CY, Akomeah M. A short, economical synthesis of 2-methoxyestradiol, an anticancer agent in clinical trials. J Org Chem. 2009;74(16):6362–6364.
Berg D, Sonsalla R, Kuss E. Concentrations of 2-methoxyoestrogens in human serum measured by a heterologous immunoassay with an 125I-labelled ligand. Acta Endocrinol (Copenh). 1983;103(2):282–288.
Barnes CM, McElrath TF, Folkman J, Hansen AR. Correlation of 2-methoxyestradiol levels in cord blood and complications of prematurity. Pediatr Res. 2010;67(5):545–550.
Pérez-Sepúlveda A, Torres MJ, Valenzuela FJ, et al. Low 2-methoxyestradiol levels at the first trimester of pregnancy are associated with the development of pre-eclampsia. Prenat Diagn. 2012;32(11):1–6.
Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51(4):593–628.
Palmer K, Saglam B, Whitehead C, Stock O, Lappas M, Tong S. Severe early-onset preeclampsia is not associated with a change in placental catechol O-methyltransferase (COMT) expression. Am J Pathol. 2011;178(6):2484–2488.
Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250.
Syvanen AC, Tilgmann C, Rinne J, Ulmanen I. Genetic polymorphism of catechol-O-methyltransferase (COMT): correlation of genotype with individual variation of S-COMT activity and comparison of the allele frequencies in the normal population and parkinsonian patients in Finland. Pharmacogenetics. 1997; 7(1):65–71.
Sata F, Yamada H, Suzuki K, et al. Functional maternal catechol-O-methyltransferase polymorphism and fetal growth restriction. Pharmacogenet Genomics. 2006;16(11):775–781.
Stanley JL, Andersson IJ, Poudel R, et al. Sildenafil citrate rescues fetal growth in the catechol-O-methyl transferase knockout mouse model. Hypertension. 2012;59(5):1021–1028.
Barnea ER, MacLusky NJ, DeCherney AH, Naftolin F. Catechol-o-methyl transferase activity in the human term placenta. Am J Perinatol. 1988;5(2):121–127.
Roten LT, Fenstad MH, Forsmo S, et al. A low COMT activity haplotype is associated with recurrent preeclampsia in a Norwegian population cohort (HUNT2). Mol Hum Reprod. 2011; 17(7):439–446.
Lim JH, Kim SY, Kim do J, et al. Genetic polymorphism of catechol-O-methyltransferase and cytochrome P450c17alpha in preeclampsia. Pharmacogenet Genomics. 2010;20(10):605–610.
Liang S, Liu X, Fan P, et al. Association between Val 158Met functional polymorphism in the COMT gene and risk of preeclampsia in a Chinese population. Arch Med Res. 2012;43(2):154–158.
Hill LD, York TP, Kusanovic JP, et al. Epistasis between COMT and MTHFR in maternal-fetal dyads increases risk for preeclampsia. PLoS One. 2011;6(1):e16681.
Ananth CV, Elsasser DA, Kinzler WL, et al. Polymorphisms in methionine synthase reductase and betaine-homocysteine S-methyltransferase genes: risk of placental abruption. Mol Genet Metab. 2007;91(1):104–110.
Sharma P, Senthilkumar RD, Brahmachari V, et al. Mining literature for a comprehensive pathway analysis: a case study for retrieval of homocysteine related genes for genetic and epigenetic studies. Lipids Health Dis. 2006;5:1.
Walker MC, Smith GN, Perkins SL, Keely EJ, Garner PR. Changes in homocysteine levels during normal pregnancy. Am J Obstet Gynecol. 1999;180(3 pt 1):660–664.
Cikot RJ, Steegers-Theunissen RP, Thomas CM, de Boo TM, Merkus HM, Steegers EA. Longitudinal vitamin and homocysteine levels in normal pregnancy. Br J Nutr. 2001;85(1):49–58.
Murphy MM, Scott JM, McPartlin JM, Fernandez-Ballart JD. The pregnancy-related decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution, or a decrease in albumin in a longitudinal study. Am J Clin Nutr. 2002;76(3):614–619.
Steegers-Theunissen RP, Wathen NC, Eskes TK, van Raaij-Selten B, Chard T. Maternal and fetal levels of methionine and homocysteine in early human pregnancy. Br J Obstet Gynaecol. 1997;104(1):20–24.
Powers RW, Evans RW, Majors AK, et al. Plasma homocysteine concentration is increased in preeclampsia and is associated with evidence of endothelial activation. Am J Obstet Gynecol. 1998; 179(6 pt 1):1605–1611.
Wang J, Trudinger BJ, Duarte N, Wilcken DE, Wang XL. Elevated circulating homocyst(e)ine levels in placental vascular disease and associated pre-eclampsia. BJOG. 2000;107(7):935–938.
D’Anna R, Baviera G, Corrado F, Ientile R, Granese D, Stella NC. Plasma homocysteine in early and late pregnancies complicated with preeclampsia and isolated intrauterine growth restriction. Acta Obstet Gynecol Scand. 2004;83(2):155–158.
Makedos G, Papanicolaou A, Hitoglou A, et al. Homocysteine, folic acid and B12 serum levels in pregnancy complicated with preeclampsia. Arch Gynecol Obstet. 2007;275(2):121–124.
Lopez-Quesada E, Vilaseca MA, Lailla JM. Plasma total homocysteine in uncomplicated pregnancy and in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2003;108(1):45–49.
Cotter AM, Molloy AM, Scott JM, Daly SF. Elevated plasma homocysteine in early pregnancy: a risk factor for the development of nonsevere preeclampsia. Am J Obstet Gynecol. 2003; 189(2):391–394.
Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol. 2006;132(2):171–196.
Valenzuela FJ, Perez-Sepulveda A, Torres MJ, Correa P, Repetto GM, Illanes SE. Pathogenesis of preeclampsia: the genetic component. J Pregnancy. 2012;2012:632732.
Shenoy V, Kanasaki K, Kalluri R. Pre-eclampsia: connecting angiogenic and metabolic pathways. Trends Endocrinol Metab. 2010;21(9):529–536.
Troisi R, Potischman N, Roberts JM, et al. Correlation of serum hormone concentrations in maternal and umbilical cord samples. Cancer Epidemiol Biomarkers Prev. 2003;12(5):452–456.
Ghorashi V, Sheikhvatan M. The relationship between serum concentration of free testosterone and pre-eclampsia. Endokrynol Pol. 2008;59(5):390–392.
Carlsen SM, Romundstad P, Jacobsen G. Early second-trimester maternal hyperandrogenemia and subsequent preeclampsia: a prospective study. Acta Obstet Gynecol Scand. 2005;84(2): 117–121.
Sowers MR, Wilson AL, Kardia SR, Chu J, Ferrell R. Aromatase gene (CYP 19) polymorphisms and endogenous androgen concentrations in a multiracial/multiethnic, multisite study of women at midlife. Am J Med. 2006;119(9 suppl 1):S23–S30.
Somner J, McLellan S, Cheung J, et al. Polymorphisms in the P450 c17 (17-hydroxylase/17,20-Lyase) and P450 c19 (aromatase) genes: association with serum sex steroid concentrations and bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2004;89(1):344–351.
Kidokoro K, Ino K, Hirose K, et al. Association between CYP19A1 polymorphisms and sex hormones in postmenopausal Japanese women. J Hum Genet. 2009;54(2):78–85.
Hertig A, Liere P, Chabbert-Buffet N, et al.. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. Am J Obstet Gynecol. 2010;203(5):477 e471–477 e479.
Czajka-Oraniec I, Simpson ER. Aromatase research and its clinical significance. Endokrynol Pol. 2010;61(1):126–134.
Simpson ER, Clyne C, Rubin G, et al. Aromatase–a brief overview. Annu Rev Physiol. 2002;64:93–127.
Conley A, Hinshelwood M. Mammalian aromatases. Reproduction. 2001;121(5):685–695.
Hong Y, Li H, Yuan YC, Chen S. Molecular characterization of aromatase. Ann N Y Acad Sci. 2009;1155:112–120.
Zhou H, Fu G, Yu H, Peng C. Transforming growth factor-beta inhibits aromatase gene transcription in human trophoblast cells via the Smad2 signaling pathway. Reprod Biol Endocrinol. 2009;7:146.
Williams MA, Woelk GB, King IB, Jenkins L, Mahomed K. Plasma carotenoids, retinol, tocopherols, and lipoproteins in preeclamptic and normotensive pregnant Zimbabwean women. Am J Hypertens. 2003;16(8):665–672.
Zhu SJ, Li Y, Li H, et al. Retinoic acids promote the action of aromatase and 17beta-hydroxysteroid dehydrogenase type 1 on the biosynthesis of 17beta-estradiol in placental cells. J Endocrinol. 2002;172(1):31–43.
Milczarek R, Sokolowska E, Hallmann A, Klimek J. The NADPH- and iron-dependent lipid peroxidation in human placental microsomes. Mol Cell Biochem. 2007;295(1–2): 105–111.
Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57(2):217–252.
Jiang B, Kamat A, Mendelson CR. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2). Mol Endocrinol. 2000;14(10):1661–1673.
Caniggia I, Winter JL. Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies—a review. Placenta. 2002;23(suppl A):S47–S57.
Tao MH, Cai Q, Zhang ZF, et al. Polymorphisms in the CYP19A1 (aromatase) gene and endometrial cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2007;16(5):943–949.
Ma CX, Adjei AA, Salavaggione OE, et al. Human aromatase: gene resequencing and functional genomics. Cancer Res. 2005; 65(23):11071–11082.
Nagar S, Walther S, Blanchard RL. Sulfotransferase (SULT) 1A1 polymorphic variants *1, *2, and *3 are associated with altered enzymatic activity, cellular phenotype, and protein degradation. Mol Pharmacol. 2006;69(6):2084–2092.
Nowell S, Falany CN. Pharmacogenetics of human cytosolic sulfotransferases. Oncogene. 2006;25(11):1673–1678.
Adjei AA, Thomae BA, Prondzinski JL, Eckloff BW, Wieben ED, Weinshilboum RM. Human estrogen sulfotransferase (SULT1E1) pharmacogenomics: gene resequencing and functional genomics. Br J Pharmacol. 2003;139(8):1373–1382.
Aksoy IA, Wood TC, Weinshilboum R. Human liver estrogen sulfotransferase: identification by cDNA cloning and expression. Biochem Biophys Res Commun. 1994;200(3):1621–1629.
Song WC, Moore R, McLachlan JA, Negishi M. Molecular characterization of a testis-specific estrogen sulfotransferase and aberrant liver expression in obese and diabetogenic C57BL/KsJ-db/db mice. Endocrinology. 1995;136(6):2477–2484.
Falany JL, Azziz R, Falany CN. Identification and characterization of cytosolic sulfotransferases in normal human endometrium. Chem Biol Interact. 1998;109(1–3):329–339.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perez-Sepulveda, A., España-Perrot, P.P., Norwitz, E.R. et al. Metabolic Pathways Involved in 2-Methoxyestradiol Synthesis and Their Role in Preeclampsia. Reprod. Sci. 20, 1020–1029 (2013). https://doi.org/10.1177/1933719113477483
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719113477483