Abstract
Rationale
Ciprofloxacin (CPFX) has been reported to inhibit cell growth and induce apoptosis in certain eukaryotic cells. The role of the mitochondrial pathway in CPFX-induced apoptosis in cultured murine sperm cells was investigated.
Methods and Results
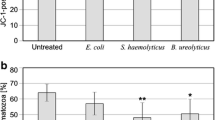
Sperm cells (5×103 cells/well) from 8-week-old NMRI male mice were cultured in 150 μL of HAM’s F10 with 25 mmol/L (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid ) HEPES and 10% human serum albumin and were incubated with 50, 100, 200, 400, and 800 µg/mL CPFX for 24 and 36 hours. Cell cytotoxicity, mitochondrial membrane potential (ΔΨM), and concentrations of caspase 3 and caspase 9 were assessed in CPFX-treated cultured murine sperm cells by MTT (3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide) , JC-1 (5, 5á, 6, 6á-tetrachloro-1, 1á, 3, 3á-tetraethylbenzimidazol-carbocyanine iodide)aggregation, and caspase 3 and caspase 9 assays, respectively. Increasing doses of CPFX showed significant cytotoxicity (EC50 = 146.73 μg/mL). Significant loss of ΔΨm was observed in sperm cells treated with ≥50 μg/mL CPFX for 36 hours. Significant increases in caspase 9 and caspase 3 concentrations were also found in cells treated with ≥50 μg/mL CPFX for 24 and 36 hours, respectively (P < .001).
Conclusions
Effects of clinically reachable doses of CPFX on cultured murine sperm cells were investigated and revealed that it may cause sperm cell toxicity by induction of apoptosis through the mitochondrial pathway in the clinically reachable concentrations.
Similar content being viewed by others
References
Menschik M, Neumuller J, Steiner CW, et al. Effects of ciprofloxacin and ofloxacin on adult human cartilage in vitro. Antimicrob Agents Chemother. 1997;41(11):2562–2565.
Sharma PC, Jain A, Jain S, Pahwa R, Yar MS. Ciprofloxacin: review on developments in synthetic, analytical, and medicinal aspects. J Enzyme Inhib Med Chem. 2010;25(4):577–589.
Kloskowski T, Gurtowska N, Drewa T. Does ciprofloxacin have an obverse and a reverse? Pulm Pharmacol Ther. 2010;23(5): 373–375.
Stahlmann R. Clinical toxicological aspects of fluoroquinolones. Toxicol Lett. 2002;127(1–3):269–277.
Khaki A, Heidari M, Ghaffari Novin M, Khaki AA. Adverse effects of ciprofloxacin on testis apoptosis and sperm parameters in rats. Iranian J Reprod Med. 2008;6(2):71–76.
Abd-Allah AR, Aly HA, Moustafa AM, Abdel-Aziz AA, Hamada FM. Adverse testicular effects of some quinolone members in rats. Pharmacol Res. 2000;41(2):211–219.
Nishikawa T, Kohjimoto Y, Nishihata M, Ebisuno S, Hara I. Synergistic antitumor effects of fleroxacin with 5-fluorouracil in vitro and in vivo for bladder cancer cell lines. Urology. Vol 74. United States, USA:2009:1370–1376.
Kamat AM, DeHaven JI, Lamm DL. Quinolone antibiotics: a potential adjunct to intravesical chemotherapy for bladder cancer. Urology. Vol 54. United States, USA:1999:56–61.
Koziel R, Szczepanowska J, Magalska A, et al. Ciprofloxacin inhibits proliferation and promotes generation of aneuploidy in Jurkat cells. J Physiol Pharmacol. 2010;61(2):233–239.
Multhaupt HA, Alvarez JC, Rafferty PA, Warhol MJ, Lackman RD. Fluoroquinolone’s effect on growth of human chondrocytes and chondrosarcomas. In vitro and in vivo correlation. J Bone Joint Surg Am. 2001;83-A Suppl 2(Pt 1):56–61.
Aranha O, Grignon R, Fernandes N, et al. Suppression of human prostate cancer cell growth by ciprofloxacin is associated with cell cycle arrest and apoptosis. Int J Oncol. 2003;22(4):787–794.
Herold C, Ocker M, Ganslmayer M, et al. Ciprofloxacin induces apoptosis and inhibits proliferation of human colorectal carcinoma cells. Br J Cancer. 2002;86(3):443–448.
Mondal ER, Das SK, Mukherjee P. Comparative evaluation of antiproliferative activity and induction of apoptosis by some fluoroquinolones with a human non-small cell lung cancer cell line in culture. Asian Pac J Cancer Prev. 2004;5(2):196–204.
Herbold BA, Brendler-Schwaab SY, Ahr HJ. Ciprofloxacin: in vivo genotoxicity studies. Mutat Res. Vol 498. Netherlands: 2001:193–205.
Holtom PD, Pavkovic SA, Bravos PD, et al. Inhibitory effects of the quinolone antibiotics trovafloxacin, ciprofloxacin, and levofloxacin on osteoblastic cells in vitro. J Orthop Res. 2000;18(5):721–727.
Lawrence JW, Claire DC, Weissig V, Rowe TC. Delayed cytotoxicity and cleavage of mitochondrial DNA in ciprofloxacin-treated mammalian cells. Mol Pharmacol. 1996;50(5):1178–1188.
Aranha O, Zhu L, Alhasan S, et al. Role of mitochondria in ciprofloxacin induced apoptosis in bladder cancer cells. J Urol. 2002; 167(3):1288–1294.
Zobeiri F, Sadrkhanlou R-A, Salami S, Ahmadi A, Mardani K. The effect of ciprofloxacin on sperm DNA damage, fertility potential and early embryonic development in mice. Veterinary Research Forum. 2012;3(2):131–135.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. May 1976;72:248–254.
Buchanan JF, Davis LJ. Drug-induced infertility. Drug Intell Clin Pharm. 1984;18(2):122–132.
Beeley L. Drug-induced sexual dysfunction and infertility. Adverse Drug React Acute Poisoning Rev. 1984;3(1):23–42.
Peirouvi T, Salami S. GnRH agonist induces apoptosis in seminiferous tubules of immature rats: direct gonadal action. Andrologia. 2010;42(4):231–235.
Yan W, Suominen J, Samson M, Jegou B, Toppari J. Involvement of Bcl-2 family proteins in germ cell apoptosis during testicular development in the rat and pro-survival effect of stem cell factor on germ cells in vitro. Mol Cell Endocrinol. 2000;165(1–2):115–129.
Boulogne B, Olaso R, Levacher C, Durand P, Habert R. Apoptosis and mitosis in gonocytes of the rat testis during foetal and neonatal development. Int J Androl. 1999;22(6):356–365.
Dunkel L, Hirvonen V, Erkkila K. Clinical aspects of male germ cell apoptosis during testis development and spermatogenesis. Cell Death Differ. 1997;4(3):171–179.
Mori C, Nakamura N, Dix DJ, et al. Morphological analysis of germ cell apoptosis during postnatal testis development in normal and Hsp 70-2 knockout mice. Dev Dyn. 1997;208(1): 125–136.
Zini A, Agarwal A. Sperm Chromatin: Biological and Clinical Applications in Male Infertility and Assisted Reproduction: Springer.
Lozano GM, Bejarano I, Espino J, et al. Relationship between caspase activity and apoptotic markers in human sperm in response to hydrogen peroxide and progesterone. J Reprod Dev. 2009;55(6): 615–621.
Taylor SL, Weng SL, Fox P, et al. Somatic cell apoptosis markers and pathways in human ejaculated sperm: potential utility as indicators of sperm quality. Mol Hum Reprod. 2004;10(11): 825–834.
Weng SL, Taylor SL, Morshedi M, et al. Caspase activity and apoptotic markers in ejaculated human sperm. Mol Hum Reprod. 2002;8(11):984–991.
Bragg PW, Handel MA. Protein synthesis in mouse spermatozoa. Biol Reprod. 1979;20(2):333–337.
Gur Y, Breitbart H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006; 20(4):411–416.
Gur Y, Breitbart H. Protein synthesis in sperm: dialog between mitochondria and cytoplasm. Mol Cell Endocrinol. 2008; 282(1–2):45–55.
Herold C, Ganslmayer M, Ocker M, et al. Overadditive antiproliferative and pro-apoptotic effects of a combination therapy on colorectal carcinoma cells. Int J Oncol. 2003;23(3): 751–756.
Kaminskyy V, Kulachkovskyy O, Stoika R. A decisive role of mitochondria in defining rate and intensity of apoptosis induction by different alkaloids. Toxicol Lett. 2008;177(3):168–181.
Wang Q, Zhao XF, Ji YL, et al. Mitochondrial signaling pathway is also involved in bisphenol A induced germ cell apoptosis in testes. Toxicol Lett. 2010;199(2):129–135.
Kalia S, Bansal MP. Regulation of apoptosis by Caspases under oxidative stress conditions in mice testicular cells: in vitro molecular mechanism. Mol Cell Biochem. 2009;322(1–2):43–52.
Kim JM, Ghosh SR, Weil AC, Zirkin BR. Caspase-3 and caspase-activated deoxyribonuclease are associated with testicular germ cell apoptosis resulting from reduced intratesticular testosterone. Endocrinology. 2001;142(9):3809–3816.
Raza A, Qawi H, Andric T, et al. Pentoxifylline, Ciprofloxacin and Dexamethasone improve the ineffective hematopoiesis in myelodysplastic syndrome patients; malignancy. Hematology. 2000;5(4):275–284.
Bergan T. Extravascular penetration of ciprofloxacin. A review. Diagn Microbiol Infect Dis. 1990;13(2):103–114.
Naber KG, Sorgel F, Kinzig M, Weigel DM. Penetration of ciprofloxacin into prostatic fluid, ejaculate and seminal fluid in volunteers after an oral dose of 750 mg. J Urol. 1993;150(5 Pt 2): 1718–1721.
Klemmt L, Scialli AR. The transport of chemicals in semen. Birth Defects Res B Dev Reprod Toxicol. 2005;74(2):119–131.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zobeiri, F., Salami, S., Sadrkhanlou, R. et al. Role of Mitochondria in Ciprofloxacin-Induced Apoptosis in Murine Sperm Cells. Reprod. Sci. 20, 1090–1095 (2013). https://doi.org/10.1177/1933719113477482
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719113477482