Abstract
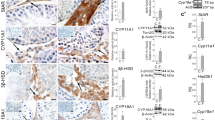
Complete suppression of the production of sperm in rats with dienogest (DNG, 40 mg/kg body weight [bw]) plus testosterone undecanoate (TU, 25 mg/kg bw), every 45 days, was found to be associated with a significant increase in germ cell apoptosis. Caspase 3 activity and expression in testis were simultaneously upregulated. Rise in the activities of caspase 8 and 9 was associated with overexpression of upstream marker proteins from extrinsic (Fas [Fatty acid synthase], FasL [Fatty acid synthase ligand], and caspase 8) and intrinsic (Bax [Bcl2-associated-x protein], Bcl2 [B-cell lymphoma 2], and caspase 9) pathways of apoptosis. Apart from the germ cells, interstitial cell apoptosis was also observed along with a decline in the number of functional Leydig cells. It is therefore concluded that complete suppression of the production of sperm with DNG + TU is facilitated mainly through the removal of precursor germ cells through apoptosis. The process is largely modulated by upregulation of upstream and downstream marker proteins from intrinsic as well as extrinsic pathway of metazoan apoptosis.
Similar content being viewed by others
References
Li J-W, Gu Y-Q. Predictors for partial suppression of spermatogenesis of hormonal male contraception. Asian J Androl. 2008; 10:723–730.
Ly LP, Liu PY, Handelsman DJ. Rates of suppression and recovery of human sperm output in testosterone-based hormonal contraceptive regimens. Hum Reprod. 2005;20:1733–1740.
Anderson RA, Wallace AM, Kicman AT, Wu FCW. Comparison between testosterone enanthate induced azoospermia and oligospermia in the male contraceptive study. IV. Suppression of endogenous testicular and adrenal androgens. Hum Reprod. 1997;12(8):1657–1662.
Misro MM, Chaki SP, Kaushik M, Nadan D. Trials for development of a once-a-month injectable male contraceptive using dienogest plus testosterone Contraception. 2009;79(6):488–497.
World Health Organization. Task force on methods for the regulation of male fertility. Rates of testosterone induced suppression to severe oligozoospermia or azoospermia in two multinational clinical studies. Int J Androl. 1995;18(3):157–165.
Matsumoto AM. Is high doses testosterone an effective male contraceptive agent? Fertil Steril. 1988;50(2):324–328.
Meena R, Misro MM, Ghosh D, Nandan D. Extended intervention time and evaluation of sperm suppression by dienogest plus testosteerone undecanoate in male rat. Contraception. 2012; 85(1):113–121.
Meena R, Misro MM, Ghosh D, Nandan D. Complete sperm suppression induced by dienogest plus testosterone undecanoate is associated with downregulation in the expression of upstream steroidogenic enzyme genes in rat testis. Contraception. 2012; 86(2):163–171.
Maheshwari A, Misro MM, Aggarwal A, Sharma RK. N-acetyl-L-cysteine modulates multiple signaling pathways to rescue male germ cells from apoptosis induced by chronic hCG administration to rats. Apoptosis. 2012;17(6):551–565.
Wu FC. Hormonal approaches to male contraception: approaching reality. Mol cell Endocrinol. 2006;250(1–2):2–7.
Anderson RA. Hormonal contraception in the male. Br Med Bull. 2000;56(3):717–728.
Manetti GJ, Honig SC. Update on male hormonal contraception: is the vasectomy in jeoparady? Int J Impot Res. 2010;22(3): 159–170.
World Health Organization. Task force on methods for the regulation of male fertility. Contraceptive efficacy of testosterone induced azoospermia and oligospermia in normal men. Fertil Steril. 1996;5:821–829.
Meriggiola MC, Bremner WJ. Androgen combination regimens for male contraception. J Androl. 1997;18(3):240–244.
Bebb RA, Anawalt BD, Christensen RB, Paulsen CA, Bremner WJ, Matsumoto AM. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. J Clin Endocrinol. 1996;81(2):757–762.
Handelsman DJ, Conway AJ, Howe CJ, Turner L, Mackey MA. Establishing the minimum effective dose and additive effects of depot progestin in suppression of human spermatogenesis by a testosterone depot. J Clin Endocrinol Metab. 1996;81(11): 4113–4121.
Meriggiola MC, Bremner WJ, Paulsen CA, et al A combined regimen of cyproterone acetate and testosterone enanthate as a potentially highly effective male contraceptive. J Clin Endocrinol Metab. 1996;81(8):3018–3023.
Meriggiola MC, Bremner WJ, Costantino A, Di Cintio G, Flamigni C. Low dose of crypreterone acetate and testosterone enanthate for contraception in men. Hum Reprod. 1998;13(5): 1225–1229.
Anawalt BD, Bebb RA, Bremner WJ, Matsumoto AM. A lower dosage levonorgestrel and testosterone combination effectively suppresses spermatogenesis and circulating gonadotropin levels with fewer metabolic effects than higher dosage combinations. J Androl. 1999;20(3):407–414.
Anawalt BD, Herbst KL, Matsumoto AM, Mulders TM, Coelingh-Bennink HJ, Bremner WJ. Desogestrel plus testosterone effectively suppresses spermatogenesis but also causes modest weight gain and high-density lipoprotein suppression. Fertil Steril. 2000;74(4):707–714.
Kamischke A, Venherm S, Ploger D, von Eckardstein S, Nieschlag E. Intramuscular testosterone undecanoate and norethisterone enanthate in a clinical trial for male contraception. J Clin Endocrinol Metab. 2001;86(1):303–309.
Kinninburg D, Anderson RA, Baired DT. Suppression of spermatogenesis with desogestrel and testosterone pellets is not enhanced by addition of finsteride. J Androl. 2001;22:88–95.
Zirkin BR, Santulli R, Awoniyi CA, Ewing LL. Maintenance of advance spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology. 1989;124:3043–3049.
Page ST, Kalhorn TF, Bremner WJ, Anawalt BD, Matusumoto AM, Amory JK. Intratesticular androgens and spermatogenesis during severe gonadotropin suppression induced by male hormonal contraceptive treatment. J Androl. 2007;28:734–741.
Meriggiola MC, Farley TMM, Mbizvo MT. A review of androgen-progestin regimens for male contraception. J Androl. 2003;24(4):466–483.
Aggarwal A, Misro MM, Maheshwari A, Sehgal N, Nandan D. Adverse effects associated with persistent stimulation of Leydig cells with hCG in vitro. Mol Reprod Dev. 2009;76(11):1076–1083.
Aggarwal A, Misro MM, Maheshwari A, Sehgal N. Differential modulation of apoptotic gene expression by NAC in Leydig cells stimulated persistently with hCG in vitro. Mol Cell Endocrinol. 2012;348(1):155–164.
Koji T. Male germ cell death in mouse testes: possible involvement of Fas and Fas ligand. Med Electron Microsc. 2001;34(4):213–222.
Giampietri C, Petrungaro S, Coluccia P, et al. Germ cell apoptosis control during spermatogenesis. Contraception. 2005;72:298–302.
Bartke A. Apoptosis of male germ cells, a generalized or a cell type-specific phenomenon? Endocrinology. 1995;136(1):3–4.
Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hsueh AJ. Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology. 1995;136(1):5–12.
Lue Y, Wang C, Cui Y, et al. Levonorgestrel enhances spermatogenesis suppression by testosterone with greater alteration in testicular gene expression in men. Biol Reprod. 2009;80:484–492.
Pentikainen V, Erkkila K, Dunkel L. Fas regulates germ cell apoptosis in the human testis in vitro. Am J Physiol. 1999;276(2 pt 1):E310–E316.
Tripathi R, Mishra DP, Shaha C. Male germ cell development: turning on the apoptotic pathways. J Reprod Immunol. 2009; 83(1):31–35.
Krajewski S, Bodrug S, Krajewska M, et al. Immunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1 in control of programmed cell death in vivo. Am J Pathol. 1995;146(6):1309–1319.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meena, R., Misro, M.M. & Ghosh, D. Complete Sperm Suppression in Rats With Dienogest Plus Testosterone Undecanoate Is Facilitated Through Apoptosis in Testicular Cells. Reprod. Sci. 20, 771–780 (2013). https://doi.org/10.1177/1933719112466305
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719112466305