Abstract
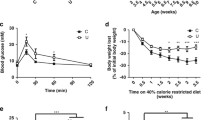
Metabolic flexibility is the body’s ability to adapt to changing energy demand and nutrient supply. Maternal undernutrition causes growth restriction at birth and subsequent obesity development. Intriguingly, metabolic flexibility is maintained due to adaptations of muscle tissue. The aim of the present study was to investigate developmental pathways of these adaptive changes. Wistar rats received standard chow at either ad libitum (AD) or 30% of ad libitum intake (UN) throughout pregnancy. At all ages, metabolic status indicated similar insulin sensitivity in AD and UN offspring despite the development of adiposity in UN offspring at weaning. Type IIA fiber size was reduced in soleus muscle of UN offspring at weaning and they had a higher percentage of type I fibers in adulthood with a concomitantly higher oxidative capacity. Plasticity of muscle was present during the postnatal period and proposes novel pathways for the dynamic development of metabolic flexibility throughout postnatal life.
Similar content being viewed by others
References
Nathanielsz PW, Thornburg KL. Fetal programming: from gene to functional systems-an overview. J Physiol. 2003;547:(pt 1)3–4.
McMillen IC, Adam CL, Mühlhäuser BS. Early origins of obesity: programming the appetite regulatory system. J Physiol. 2005;565(pt 1):9–17.
Phillips DIW, Jones A. Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses. J Physiol. 2005;572(pt 1):45–50.
Sayer AA, Cooper C. Fetal programming of body composition and musculoskeletal development. Early Hum Dev. 2005;81(9): 735–744.
Symonds ME, Stephenson T, Gardner DS, Budge H. Long-term effects of nutritional programming of the embryo and fetus: mechanisms and critical windows. Reprod Fertil Dev. 2007; 19(1):53–63.
Green A, Rozance P, Limesand S. Consequences of a compromised intrauterine environment on islet function. J Endocrinol. 2010; 205(3):211–224.
Thompson NM, Norman AM, Donkin SS, et al. Prenatal and postnatal pathways to obesity: different underlying mechanisms, different metabolic outcomes. Endocrinology. 2007;148(5):2345–2354.
Miles JL, Huber K, Thompson NM, Davison M, Breier BH. Moderate daily exercise activates metabolic flexibility to prevent prenatally induced obesity. Endocrinology. 2009;150(1):179–186.
Huber K, Miles JL, Norman AM, Thompson NM, Davison M, Breier BH. Prenatally induced changes in muscle structure and metabolic function facilitate exercise-induced obesity prevention. Endocrinology. 2009;150(9):4135–4144.
Bauer R, Gedrange T, Bauer K, Walter B. Intrauterine growth restriction induces increased capillary density and accelerated type I fiber maturation in newborn pig skeletal muscles. J Perinat Med. 2006;34(3):235–242.
Wilson SJ, Ross JJ, Harris AJ. A critical period for formation of secondary myotubes defined by prenatal undernourishment in rats. Development. 1988;102(4):815–821.
Pagel-Langenickel I, Bao J, Pang L, Sack MN. The role of mitochondria in the pathophysiology of skeletal muscle insulin resistance. Endocr Rev. 2010;31(1):25–51.
Punkt K, Naupert A, Asmussen G. Differentiation of rat skeletal muscle fibres during development and ageing. Acta Histochem. 2004;106(2):145–154.
Zhu MJ, Ford SP, Means WJ, Hess BW, Nathanielsz PW, Du M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol. 2006;575(pt 1):241–250.
Bedi KS, Birzgalis AR, Mahon M, Smart JL, Wareham AC. Early life undernutrition in rats. 1. Quantitative histology of skeletal muscles from underfed young and refed adult animals. Br J Nutr. 1982; 47(3):417–431.
Storlien L, Oakes ND, Kelley DE. Metabolic flexibility. Proc Nutr Soc. 2004;63(2):363–368.
Phielix E, Mensink M. Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol Behav. 2008;94(2):252–258.
Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288(1): R91–R96.
Lane RH, Maclennan NK, Daood MJ, et al. IUGR alters postnatal rat skeletal muscle peroxisome proliferator-activated receptor-(gamma) coactivator 1 gene expression in a fibre-specific manner. Pediatric Res. 2003;53(6):994–1000.
Roehrig KL, Allred JB. Direct enzymatic procedure for the determination of liver glycogen. Anal Biochem. 1974;58(2): 414–421.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259): 680–685.
Newsholme EA, Crabtree B. Maximum catalytic activity of some key enzymes in provision of physiologically useful information about metabolic fluxes. J Exp Zool. 1986;239(2): 159–167.
Woodall SM, Breier BH, Johnston BM, Gluckman PD. A model of intrauterine growth retardation caused by chronic maternal undernutrition in the rat: effects on the somatotrophic axis and postnatal growth. J Endocrinol. 1996;150(2):231–242.
Jahan S, Zinnat R, Hassan Z, Biswas KB, Habib SH. Gender differences in serum leptin concentrations from umbilical cord blood of newborn infants born to nondiabetic, gestational diabetic and type-2 diabetic mothers. Int J Diabetes Dev Ctries. 2009;29(4):155–158.
Nakatani T, Nakashima T, Kita T, et al. Cell size and oxidative enzyme activity of different types of fibers in different regions of the rat plantaris and tibialis anterior muscles. Jpn J Physiol. 2000;50(4):413–418.
Lunde IG, Ekmark M, Rana ZA, Buonanno A, Gundersen K. PPARdelta expression is influenced by muscle activity and induces slow muscle properties in adult rat muscles after somatic gene transfer. J Physiol. 2007;582(pt 3):1277–1287.
Krechowec SO, Vickers M, Gertler A, Breier BH. Prenatal influences on leptin sensitivity and susceptibility to diet-induced obesity. J Endocrinol. 2006;189(2):355–363.
Vickers MH. Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes. 2007;14(1): 17–22.
Vickers MH, Gluckman PD, Coveny AH, et al. The effect of neonatal treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149(4):1906–1913.
Briana DD, Malamitsi-Puchner A. Intrauterine growth restriction and adult disease: the role of adipocytokines. Eur J Endocrinol. 2009;160(3):337–347.
Yu T, Luo G, Zhang L, Wu J, Zhang H, Yang G. Leptin promotes proliferation and inhibits differentiation in porcine skeletal myoblasts. Biosci Biotechnol Biochem. 2008;72(1):13–21.
Rooney K, Ozanne SE. Maternal over-nutrition and offspring obesity predisposition: targets for preventative interventions. Int J Obesity. 2011;35(7):1–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Norman, A.M., Miles-Chan, J.L., Thompson, N.M. et al. Postnatal Development of Metabolic Flexibility and Enhanced Oxidative Capacity After Prenatal Undernutrition. Reprod. Sci. 19, 607–614 (2012). https://doi.org/10.1177/1933719111428519
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719111428519