Abstract
Objective
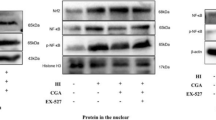
This study investigated the hypothesis that ceftriaxone preconditioning ameliorates brain damage in neonatal animals through glutamate transporter 1 (GLT-1) upregulation.
Study design
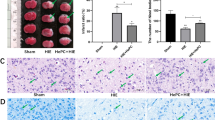
Sprague Dawley rats were pretreated with ceftriaxone, erythromycin, minocycline, or saline for 5 consecutive days starting from postnatal day 2 (P2), and GLT-1/glutamate-aspartate transporter (GLAST) messenger RNA (mRNA) and protein levels were examined in the P7 brains. After ceftriaxone or saline preconditioning, the P7 rats underwent hypoxic-ischemic (H-I) procedure or sham operation. One week after the procedure (P14), hematoxylin-eosin staining, microtubule-associated protein 2 (MAP-2) immunostaining, and transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay were used to examine neuronal damage and possible neurotoxicity.
Results
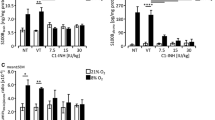
Repeated ceftriaxone injections significantly increased GLT-1 mRNA and protein levels but not GLAST. Following such treatment and H-I procedure, the MAP-2-positive area increased and TUNEL-positive cells decreased. Conclusion: Antenatal ceftriaxone may help to provide neuroprotection in the immature brain and become a new prophylactic strategy to reduce neonatal encephalopathy in clinical perinatal medicine.
Similar content being viewed by others
References
Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7(1):56–64.
McLean C, Ferriero D. Mechanisms of hypoxic-ischemic injury in the term infant. Semin Perinatol. 2004;28(6):425–432.
Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Rev. 2004;45(3):250–265.
Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1(8):623–634.
Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105.
Tanaka K, Watase K, Manabe T, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276(5319):1699–1702.
Rothstein JD, Dykes-Hoberg M, Pardo CA, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686.
Rothstein JD, Jin L, Dykes-Hoberg M, Kuncl RW. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993;90(14):6591–6595.
McDonald JW, Silverstein FS, Johnston MV. Neurotoxicity of N-methyl-D-aspartate is markedly enhanced in developing rat central nervous system. Brain Res. 1988;459(1):200–203.
Tremblay E, Roisin MP, Represa A, Charriaut-Marlangue C, Ben-Ari Y. Transient increased density of NMDA binding sites in the developing rat hippocampus. Brain Res. 1988;461(2):393–396.
McDonald JW, Johnston MV, Young AB. Ontogeny of the receptors comprising the NMDA receptor complex. Soc Neurosci Abstr. 1989;15(3):198.
Represa A, Tremblay E, Ben-Ari Y. Transient increase of NMDA-binding sites in human hippocampus during development. Neurosci Lett. 1989;99(1–2):61–66.
Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci. 1997;17(21):8363–8375.
Kugler P, Schleyer V. Developmental expression of glutamate transporters and glutamate dehydrogenase in astrocytes of the postnatal rat hippocampus. Hippocampus. 2004;14(8):975–985.
McDonald JW, Silverstein FS, Johnston MV. MK-801 protects the neonatal brain from hypoxic-ischemic damage. Eur J Pharmacol. 1987;140(3):359–361.
Olney JW, Ikonomidou C, Mosinger JL, Frierdich G. MK-801 prevents hypobaric-ischemic neuronal degeneration in infant rat brain. J Neurosci. 1989;9(5):1701–1704.
Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74.
Stefovska V, Czuczwar M, Smitka M, et al. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64(4):434–445.
Rothstein JD, Patel S, Regan MR, et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77.
Kon C, Soon-Tae L, Dong-In S, et al. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke. 2007;38(1):177–182.
Verma R, Mishra V, Sasmal D, Raghubir R. Pharmacological evaluation of glutamate transporter 1 (GLT-1) mediated neuroprotection following cerebral ischemia/reperfusion injury. Eur J Pharmacol. 2010;638(1–3):65–71.
Hagberg H, Bona E, Gilland E, Puka-Sundvall M. Hypoxia-ischemia model in the 7-day-old rat: possibilities and shortcomings. Acta Paediatr Suppl. 1997;442:85–88.
Brambrink AM, Koerner IP, Diehl K, Strobel G, Noppens R, Kempski O. The antibiotic erythromycin induces tolerance against transient global cerebral ischemia in rats (pharmacologic preconditioning). Anesthesiology. 2006;104(6):1208–1215.
Fox C, Dingman A, Derugin N, et al. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2005;25(9):1138–1149.
Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–141.
Kitagawa K, Matsumoto M, Ninobe M, et al. Microtubule-associated protein 2 as a sensitive marker for cerebral ischemic damage-immunohistochemical investigation of dendritic damage. Neuroscience. 1989;31(2):401–411.
Tomimatsu T, Fukuda H, Kanagawa T, Mu J, Kanzaki T, Murata Y. Effects of hyperthermia on hypoxic-ischemic brain damage in the immature rat: its influence on caspase-3–like protease. Am J Obstet Gynecol. 2003;188(3):768–773.
Nizzardo M, Nardini M, Ronchi D, et al. Beta-lactam antibiotic offers neuroprotection in a spinal muscular atrophy model by multiple mechanisms. Exp Neurol 2011;229(2):214–225.
Goodman LS, Hardman JG, Limbird LE, et al. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill; 2001.
Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(ll):978–985.
Nau R, Prange HW, Muth P, et al. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother. 1993;37(7):1518–1524.
Kafetzis DA, Brater DC, Fanourgakis JE, Voyatzis J, Georgakopoulos P. Ceftriaxone distribution between maternal blood and fetal blood and tissues at parturition and between blood and milk postpartum. Antimicrob Agents Chemother. 1983;23(6):870–873.
Sidhu S, Tuor UI, Del Bingio MR. Nuclear condensation and fragmentation following cerebral hypoxia-ischemia occurs more frequently in immature than older rats. Neurosci Lett. 1997;223(2):129–132.
Hu BR, Liu CL, Ouyang Y, Blomgren K, Siesjö BK. Involvement of caspase-3 in cell death after hypoxic-ischemic declines during brain maturation. J Cereb Blood Flow Metab. 2000;20(9):1294–1300.
Fukuda H, Tomimatsu T, Watanabe N, et al. Post-ischemic hypothermia blocks caspase-3 activation in the newborn rat brain after hypoxia-ischemia. Brain Res. 2001;910(1–2):187–191.
Ikeda T. Stem cells and neonatal brain injury. Cell Tissue Res. 2008;331(1):263–269.
Brooks WJ, Sarkisian M, Yang Y, Hori A, Helmers SL, Mikati M. Effect of chronic administration of NMDA antagonists on synaptic development. Synapse. 1997;26(2):104–113.
Tanaka K. Antibiotics rescue neurons from glutamate attack. Trends Mol Med. 2005;11(6):259–262.
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136.
Hagberg H, Dammann O, Mallard C, Leviton A. Preconditioning and the developing brain. Semin Perinatol. 2004;28(6):389–395.
Gidday JM, Fitzgibbons JC, Shah AR, Park TS. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett. 1994;168(1–2):221–224.
Ikeda T, Ikenoue T, Xia XY, Xia YX. Important role of 72-kd heat shock protein expression in the endothelial cell in acquisition of hypoxic-ischemic tolerance in the immature rat. Am J Obstet Gynecol. 2000;182(2):380–386.
Gustavsson M, Anderson MF, Mallard C, Hagberg H. Hypoxic preconditioning confers long-term reduction of brain injury and improvement of neurological ability in immature rats. Pediatr Res. 2005;57(2):305–309.
Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438(7071):1167–1171.
Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22(2):455–463.
Follett PL, Deng W, Dai W, et al. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci. 2004; 24(18):4412–4420.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mimura, K., Tomimatsu, T., Minato, K. et al. Ceftriaxone Preconditioning Confers Neuroprotection in Neonatal Rats Through Glutamate Transporter 1 Upregulation. Reprod. Sci. 18, 1193–1201 (2011). https://doi.org/10.1177/1933719111410710
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719111410710