Abstract
Objective
Urocortin 2 (Ucn2) and urocortin 3 (Ucn3) are new members of the corticotrophin-releasing hormone (CRH) family of peptides expressed and localized in human placenta. In the current study, we aimed to asses whether hypoxia affects placental Ucn2/Ucn3 messenger RNA (mRNA) expression and protein localization in physiological or pathological hypoxia and to evaluate whether the effect is modulated by the hypoxia-inducible factor 1α (HIF-1α).
Methods
Early first-trimester placental specimens from elective termination of pregnancy were used for villous explants and term placental tissue were used for primary cell cultures. The samples were incubated under different oxygen conditions; parallel sets exposed to hypoxia re-oxygenation (HR). Dimethyloxalylglycine (DMOG), an HIF-1α stabilizer, was used to mimic the effects of hypoxia in villous explants. Real-time polymerase chain reaction (PCR) and immunohystochemistry were performed on early pregnancy and preeclamptic (PE) placentae. mRNA levels were measured on villous explants and cell cultures incubated under different oxygen and reagent conditions.
Results
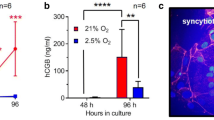
Both Ucn2 and Ucn3 mRNA expression was significantly higher at 6 to 9 weeks of gestation than 10 to 12 wks and in primary trophoblast cell cultures and explants exposed to low O2 tension (3%) compared to 20% O2. Strong Ucn2/Ucn3 immunoreactivity was present in trophoblast villi from 6 weeks placentae. Ucn2 immunostaining was stronger in early PE (E-PE) samples relative to controls whereas Ucn3 showed stronger immunoreactivity in late-PE (L-PE) placentae. Only Ucn2 transcript levels increased in HR explants. Ucn2 and Ucn3 expression by first-trimester explants was significantly greater in the presence of DMOG. All PE placentae expressed significantly higher Ucn2 and Ucn3 mRNA compared to controls.
Discussion
Placental Ucn2 and Ucn3 expression is sensitive to O2 tensions and mediated by HIF-1α. During early pregnancy, Ucn2/Ucn3 may influence trophoblast proliferation and establishment of pregnancy. In PE placentae, the increased expression of both peptides may reflect a response to the oxidative stress.
Similar content being viewed by others
References
Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7(5):605–611.
Lewis K, Li C, Perrin MH, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;19;98(13):7570–7575.
Reyes TM, Lewis K, Perrin MH, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98(5):2843–2848.
Wetzka B, Sehringer B, Schäfer WR, et al. Expression patterns of CRH, CRH receptors, and CRH binding protein in human gestational tissue at term. Exp Clin Endocrinol Diabetes. 2003;111(3): 154–161.
Imperatore A, Florio P, Torres PB, et al. Urocortin 2 and urocortin 3 are expressed by the human placenta, deciduas, and fetal membranes. Am J Obstet Gynecol. 2006;195(1):288–295.
Janatpour MJ, Utset MF, Cross JC, et al. A repertoire of differentially expressed transcription factors that offers insight into mechanisms of human cytotrophoblast differentiation. Dev Genet. 1999;25(2):146–157.
Kingdom JC, Kaufmann P, Jauniaux E. Oxygen and placental vascular development. Adv Exp Med Biol. 2000;474:259–275.
Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80(2):283–285.
Hung TH, Charnock-Jones DS, Skepper JN, Burton GJ. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. 2004;164(3):1049–1061.
Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97(2):540–550.
Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(suppl A): S25–S30.
Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet. 1999;354(9181):788–789.
Walker JJ. Pre-eclampsia. Lancet. 2000;356(9237): 1260–1265 [Review].
Caniggia I, Mostachfi H, Winter J, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest. 2000;105(5):577–587.
Metzen E, Ratcliffe PJ. HIF hydroxylation and cellular oxygen sensing. Biol Chem. 2004;385(3–4):223–230.
Ten VS, Pinsky DJ. Endothelial response to hypoxia: physiologic adaptation and pathologic dysfunction. Curr Opin Crit Care. 2002;8(3):242–250.
Chen A, Blount A, Vaughan J, Brar B, Vale W. Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: localization, basal expression in corticotropin-releasing factor receptor (CRFR) 1- and CRFR2-null mice, and regulation by glucocorticoids. Endocrinology. 2004;145(5):2445–2457.
Jain V, Longo M, Ali M, Saade GR, Chwalisz K, Garfield RE. Expression of receptors for corticotropin-releasing factor in the vasculature of pregnant rats. J Soc Gynecol Investig. 2000;7(3): 153–160.
Mackay KB, Stiefel TH, Ling N, Foster AC. Effects of a selective agonist and antagonist of CRF2 receptors on cardiovascular function in the rat. Eur J Pharmacol. 2003;469(1–3):111–115.
Coste SC, Kesterson RA, Heldwein KA, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24(4):403–409.
Gardiner SM, March JE, Kemp PA, Bennett T. A comparison between the cardiovascular actions of urocortin 1 and urocortin 2 (stresscopin-related peptide) in conscious rats. J Pharmacol Exp Ther. 2007;321(1):221–226.
Chanalaris A, Lawrence KM, Stephanou A, et al. Protective effects of the urocortin homologues stresscopin (SCP) and stresscopin-related peptide (SRP) against hypoxia/reoxygenation injury in rat neonatal cardiomyocytes. J Mol Cell Cardiol. 2003;35(10):1295–1305.
Tao J, Zhang Y, Soong TW, Li S. Urocortin II inhibits the apoptosis of mesenteric arterial smooth muscle cells via L-type calcium channels in spontaneously hypertensive rats. Cell Physiol Biochem. 2006;17(3–4):111–120.
Takahashi K, Totsune K, Murakami O, et al. Expression of urocortin III/stresscopin in human heart and kidney. J Clin Endocrinol Metab. 2004;89(4):1897–1903.
Florio P, Franchini A, Reis FM, Pezzani I, Ottaviani E, Petraglia F. Human placenta, chorion, amnion and decidua express different variants of corticotropin-releasing factor receptor messenger RNA. Placenta. 2000;21(1):32–37.
Murphy DJ, Stirrat GM. Mortality and morbidity associated with early-onset preeclampsia. Hypertens Pregnancy. 2000;19(2):221–231.
von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–148.
ACOG Committee on Practice Bulletin—Obstetric. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99(1):159–167.
Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss III JF. Purification, characterization and in vitro differentiation of cytotrophoblasts from human term placenta. Endocrinology. 1986;118(4):1567–1582.
Sun K, Yang K, Challis JRG. Differential regulation of 11 beta-hydroxysteroid dehydrogenase type 1 and 2 by nitric oxide in cultured human placental trophoblast and chorionic cell preparation. Endocrinology. 1997;138(11):4912–4920.
Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472.
Ietta F, Wu Y, Winter J, et al. Dynamic HIF-1alpha regulation during human placental development. Biol Reprod. 2006;75(1): 112–121.
Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxiareoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90(12):1274–1281.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408.
Ietta F, Wu Y, Romagnoli R, et al. Oxygen regulation of macrophage migration inhibitory factor in human placenta. Am J Physiol Endocrinol Metab. 2007;292(1):E272–E280.
Banerjee S, Smallwood A, Moorhead J, et al. Placental expression of interferon-gamma (IFN-gamma) and its receptor IFN-gamma R2 fail to switch from early hypoxic to late normotensive development in preeclampsia. Clin Endocrinol Metab. 2005;90(2): 944–952.
Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. Clin Endocrinol Metab. 2001;86(6): 2505–2512.
Munaut C, Lorquet S, Pequeux C, et al. Hypoxia is responsible for soluble vascular endothelial growth factor receptor-1 (VEGFR-1) but not for soluble endoglin induction in villous trophoblast. Hum Reprod. 2008;23(6):1407–1415.
Nevo O, Soleymanlou N, Wu Y, et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1085–R1093.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imperatore, A., Rolfo, A., Petraglia, F. et al. Hypoxia and Preeclampsia: Increased Expression of Urocortin 2 and Urocortin 3. Reprod. Sci. 17, 833–843 (2010). https://doi.org/10.1177/1933719110373147
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719110373147