Abstract
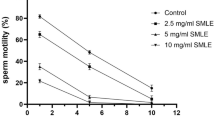
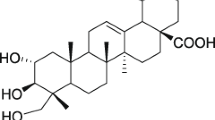
The seed extracts of Madhuca latifolia were reported to have spermicidal activity. The current investigation identified the spermicidal component of the extracts and evaluated its spermicidal potential in vitro. As characterized by infrared, mass, and nuclear magnetic resonance (NMR) spectral analyses, Mi-saponin A (MSA) was found to be the most potent component among a mixture of saponins. The mean effective concentrations of MSA that induced irreversible immobilization were 320 μg/mL for rat and 500 μg/mL for human sperm, as against the respective concentrations of 350 and 550 μg/mL of nonoxynol 9 (N-9). The mode of spermicidal action was evaluated by a battery of tests including (a) double fluoroprobe staining for sperm viability, (b) hypoosmotic swelling test and, assays for 5’ nucleotidase and acrosin for physiological integrity of sperm plasma membrane, (c) scanning and transmission electron microscopy for sperm membrane ultrastructure, and (d) plasma membrane lipid peroxidation (LPO). The observations, taken together, were interpreted to mean that the spermicidal effect of MSA involved increased membrane LPO leading to structural and functional disintegration of sperm plasma membrane and acrosomal vesicle. A comparative in vitro cytotoxicity study in human vaginal keratocyte (Vk2/E6E7) and endocervical (End/E6E7) cell lines demonstrated that the 50% cell cytotoxicity (CC50) values, and consequently the safety indices, for MSA were ≥ 8-fold higher as compared to those of N-9. In conclusion, MSA is a potent spermicidal molecule that may be explored further for its suitability as an effective component of vaginal contraceptive.
Similar content being viewed by others
References
World Health Organization. Abortion: A Tabulation of Available Data on the Frequency and Mortality of Unsafe Abortion. 3rd ed. Geneva, Switzerland: World Health Organization; 1997.
In: Thompson TG., Gerberding JL., Sondik EK., eds. Chartbook on Trends in the Health of Americans. Washington, DC, USA: US Government Printing Office; 2004.
Gupta G. Microbicidal spermicide or spermicidal microbicide? Eur J Contracept Reprod Health Care. 2005;10(4):212–218.
Farnsworth NR, Waller DP. Current status of plant products reported to inhibit sperm. In: Zatuchni GI, ed. Research Frontiers in Fertility Regulation. Evanston, Illinois: Northwestern University. 1982:2(1):1–16.
Setty BS, Kamboj VP, Khanna NM. Screening of Indian Plants for biological activity Part. VII. Spermicidal activity of Indian plants. Indian J Exp Biol. 1977;15(3):231–232.
Setty BS, Kamboj VP, Garg HS, Khanna NM. Spermicidal potential of saponins isolated from Indian medicinal plants. Contraception. 1976;14(5):571–578.
Kitagawa J, Inada A, Yosioka I. Saponin and Sapogenol XII. MSA and Mi-saponin B, Two major bisdesmosides from the seed kernels of. Madhuca longifolia. Chem Pharm Bull. 1975; 23(10):2268–2278.
Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57(4):847–855.
World Health Organization. Laboratory Manual for the Examination of Human Semen and Cervical Mucus Interaction. 4th ed. New York, NY: Cambridge University Press; 1999.
Khillare B, Shrivastav TG. Spermicidal activity of Azadirachta indica (neem) leaf extract. Contraception. 2003;68(3):225–229.
Flajshans M, Cosson J, Rodina M, Linhart O. The application of image cytometry to viability assessment in dual fluorescence-stained fish spermatozoa. Cell Biol. Int. 2004; 28(12):955–959.
Pal D, Chakraborty P, Ray HN, Pal BC, Mitra D, Kabir SN. Acaciaside-B-enriched fraction of Acacia auriculiformis is a prospective spermicide with no mutagenic property. Reproduction. 2009;138(3):453–62.
Jayendran RS, VanderVen HH, Perez-Palaez M, Carbo BG, Zaneveld LJD. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70(1):219–228.
Wilborn WH, Flowers CE, Hyde BM. Ultrastructure of endometrium of postmenopausal patients on two modes of hormonal therapy: estrone sulfate with or without MPA. Contracept Deliv Syst. 1984;4(1):71–85.
Souada K, Alib S, Mounirb A, Mounira TM. Spermicidal activity of extract from. Cestrum parqui. Contraception. 2007; 75(2):152–156.
Heppel LA, Hilmoe RJ. 5’nucleotidase of seminal plasma. In: Colowick SP., Kaplan NO., eds. Methods Enzymol. New York, NY: Academic Press; 1955;2:547–549.
Chakrabarti K, Pal S, Bhattacharyya AK. Sperm immobilization activity of Allium sativum L. and other plant extracts. Asian J Androl. 2003;5(2):131–135.
Chen PS, Toribara TY, Warner H. Micro determination of phosphorus. Anal Chem. 1956;28(11):1756–1758.
Breden TG, Berg R, Plotka ED. Effects of substrate and separation method on acrosin amidase measurements. J Androl. 1996;17(4):443–448.
Buege JA, Aust SD. Microsomal lipid peroxidation. In: Fleischer S., Packer L., eds. Methods Enzymol. New York, NY; Academic Press; 1978; 52:302–310.
Suleiman SA, Ali ME, Zaki ZM, El-Malik EM, Nasr MA. LPO and human sperm motility: protective role of vitamin E. J Androl. 1996;17(5):530–537.
Catalone BJ, Catalone TMK, Budgeon LR, et al. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob Agents Chemother. 2004;48(5):1837–1847.
Flesch FM, Gadella BM. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta. 2000;1469(3):197–235.
Sharma SK, SaiRam M, Ilvazhagan G, Devendra K, Shivaji SS, Selvamurthy W. Mechanism of action of NIM-76: a novel vaginal contraceptive from neem oil. Contraception. 1996;54(6):373–378.
Strater N. Ecto-5′-nucleotidase: structure function relationships. Purinergic Signal. 2006;2(2):343–350.
Baba T, Niida Y, Michikawa Y, et al. An acrosomal protein, sp32, in mammalian sperm is a binding protein specific for two proacrosins and an acrosin intermediate. J Biol Chem. 1994; 269(13):10133–10140.
Jonge CDJ, Tarchala SM, Rawlins RG, Binor Z, Radwanska E. Acrosin activity in human spermatozoa in relation to semen quality and in-vitro fertilization. Hum Reprod. 1993;8(2):253–257.
Rhemrev JP, Vermeiden JP, Haenen GR, DeBruijne JJ, Rekers-Mombarg LT, Bast A. Progressively motile human spermatozoa are well protected against in vitro lipid peroxidation imposed by induced oxidative stress. Andrologia. 2001;33(3):151–158.
Nandi B, Roy S, Bhattacharya S, SinhaBabu SP. Free radicals mediated membrane damage by the saponins acaciaside A and acaciaside B. Phytother Res. 2004;18(3):191–194.
Doncel GF, Chandra N, Fichorova RN. Preclinical assessment of the proinflammatory potential of microbicide candidates. J Acquir Immune Defic Syndr. 2004;37(suppl 3):S174–S180.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saha, P., Majumdar, S., Pal, D. et al. Evaluation of Spermicidal Activity of Mi-Saponin A. Reprod. Sci. 17, 454–464 (2010). https://doi.org/10.1177/1933719110361378
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719110361378