Abstract
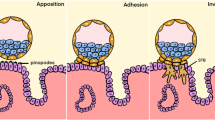
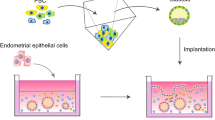
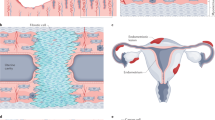
Several in vitro models that attempt to replicate the intraperitoneal environment have been developed to study the pathogenesis of endometriosis. The chicken chorioallantotic membrane has been used, but it has not been well characterized and may introduce some species specific variables. In vitro models using human tissues include amniotic membrane, human peritoneal explants, and cell culture monolayers. These models have been used to qualitatively, quantitatively, and temporally assess attachment of endometrial cells to peritoneal mesothelial and subsequent transmesothelial invasion. These models have also been used to assess the role of cytokines in the development of the early endometriotic lesion. Two- and three dimensional invasion chamber models have been utilized to assess endometrial cell interactions with peritoneal mesothelial cells and the extracellular matrix. Invasion models are also useful to evaluate novel therapeutic approaches. This review will focus on the above models to assist reproductive scientists interested in the pathogenesis of endometriosis.
Similar content being viewed by others
References
Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469.
Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64(2):151–154.
Liu DT, Hitchcock A. Endometriosis: its association with retrograde menstruation, dysmenorrhoea and tubal pathology. Br J Obstet Gynaecol. 1986;93(8): 859–862.
Keettel WC, Stein RJ. The viability of the cast-off menstrual endometrium. Am J Obstet Gynecol. 1951;61(2):440–442.
Mungyer G, Willemsen WN, Rolland R, et al. Cell of the mucous membrane of the female genital tract in culture: a comparative study with regard to the histogenesis of endometriosis. In Vitro Cell Dev Bio. 1987;23(2):111–117.
Ridley JH, Edwards IK. Experimental endometriosis in the human. Am J Obstet Gynecol. 1958;76(4):783–790.
Olive DL, Henderson DY. Endometriosis and mullerian anomalies. Obstet Gynecol. 1987;69(3 pt 1):412–415.
Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154(1):39–43.
Jenkins S, Olive DL, Haney AF. Endometriosis: pathogenetic implications of the anatomic distribution. Obstet Gynecol. 1986;67(3):335–338.
Scher C, Haudenschild C, Klagsbrun M. The chick chorioallantoic membrane as a model system for the study of tissue invasion by viral transformed cells. Cell. 1976;8(3):373–382.
Armstrong PB, Quigley JP, Sidebottom E. Transepithelial invasion and intramesenchymal infiltration of the chick embryo chorioallantois by tumor cell lines. Cancer Res. 1982;42(5):1826–1837.
Maas JW, Groothuis PG, Dunselman GA, de Goeij AF, Struijker-Boudier HA, Evers JL. Development of endometriosis-like lesions after transplantation of human endometrial fragments onto the chick embryo chorioallantoic membrane. Hum Reprod. 2001;16(4):627–631.
Nap AW, Groothuis PG, Demir AY, et al. Tissue integrity is essential for ectopic implantation of human endometrium in the chicken chorioallantoic membrane. Hum Reprod. 2003;18(1):30–34.
Giannopoulou E, Katsoris P, Hatziapostolou M, et al. X-rays modulate extracellular matrix in vivo. Int J Cancer. 2001;94(5):690–698.
Nap AW, Dunselman GA, Griffioen AW, Mayo KH, Evers JL, Groothuis PG. Angiostatic agents prevent the development of endometriosis-like lesions in the chicken chorioallantoic membrane. Fertil Steril. 2005;83(3):793–795.
Oosterlynck DJ, Meuleman C, Waer M, Koninckx PR, Vandeputte M. Immunosuppressive activity of peritoneal fluid in women with endometriosis. Obstet Gynecol. 1993;82(2):206–212.
Liotta LA, Lee CW, Morakis DJ. New method for preparing large surfaces of intact human basement membrane for tumor invasion studies. Cancer Lett. 1980;11(2):141–152.
van der Linden PJ, de Goeij AF, Dunselman GA, Erkens HW, Evers JL. Endometrial cell adhesion in an in vitro model using intact amniotic membranes. Fertil Steril. 1996;65(1): 76–80.
La Rocca P, Rheinwald J. Coexpression of simple epithelia keratin and vimentin by human mesothelium and meso-thelioma in vivo and in culture. Cancer Res. 1984;44(7): 2991–2995.
Wild RA, Zhang RJ, Medders D. Whole endometrial fragments form characteristics of in vivo endometriosis in a mesothelial cell co-culture system: an in vitro model for the study of the histogenesis of endometriosis. J Soc Gynecol Investig. 1994;1(1):65–68.
Witz CA, Montoya-Rodriguez IA, Miller DM, Schneider BG, Schenken RS. Mesothelium expression of integrins in vivo and in vitro. J Soc Gynecol Invest. 1998;5(2): 87–93.
van der Linden PJ, de Goeij AF, Dunselman GA, Erkens HW, Evers JL. Amniotic membrane as an in vitro model for endometrium-extracellular matrix interactions. Gynecol Obstet Invest. 1998;45(1):7–11.
Witz CA, Montoya IA, Schenken RS. Whole explants of peritoneum and endometrium: a novel model of the early endometriosis lesion. Fertil Steril. 1999;71(1):56–60.
Groothuis P, Koks CA, de Goeij AF, Dunselman GA, Arends JW, Evers JL. Adhesion of human endometrial fragments to peritoneum in vitro. Fertil Steril. 1999;71(6):1119–1124.
Koks CA, Groothuis PG, Dunselman GA, de Goeij AF, Evers JL. Adhesion of shed menstrual tissue in an in-vitro model using amnion and peritoneum: a light and electron microscopic study. Hum Reprod. 1999;14(3):816–822.
Witz CA, Thomas MR, Montoya-Rodriguez IA, Nair AS, Centonze VE, Schenken RS. Short-term culture of peritoneum explants confirms attachment of endometrium to intact peritoneal mesothelium. Fertil Stertil. 2001;75(2):385–390.
Witz CA, Cho S, Centonze VE, Montoya-Rodriguez IA, Schenken RS. Time series analysis of transmesothelial invasion by endometrial stromal and epithelial cells using three-dimensional confocal microscopy. Fertil Steril. 2003;79(suppl 1):770–778.
Witz CA, Allsup KT, Montoya-Rodriguez IA, Vaughn SL, Centonze VE, Schenken RS. Culture of menstrual endometrium with peritoneal explants and mesothelial monolayers confirms attachment to intact mesothelial cells. Hum Reprod. 2002;17(11):2832–2838.
Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril. 2005;84(1):16–21.
Griffith J, Lui YG, Binkley PA, Tekmal RR, Holden AEC, Schenken RS. Menstrual endometrial cells from women with endometriosis demonstrate increased adherence to peritoneal cells and increased expression of CD44 splice variants. Fertil Steril. IN PRESS.
Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function and association with the malignant process. Adv Cancer Res. 1997;71:241–319.
Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61(7):1303–1313.
Heldin P, Pertoff H. Synthesis and assembly of the hyaluronan-coating coats around normal human mesothelial cells. Exp Cell Res. 1993;208(2):422–429.
Cannistra SA, Kansas GS, Niloff J, DeFranzo B, Kim Y, Ottensmeier C. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res. 1993;53(16):3830–3838.
Lessan K, Aguiar DJ, Oegema T, Siebenson L, Skubitz AP. CD44 and beta 1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol. 1999;154(5):1525–1537.
Witz CA, Takahashi A, Montoya-Rodriguez IA, Cho S, Schenken RS. Expression of the a2B1 and a3B1 integrins at the surface of mesothelial cells: a potential attachment site of endometrial cells. Fertil Steril. 2000;74(3):579–584.
Tietze L, Borntraeger J, Klosterhalfen B, et al. Expression and function of beta(1) and beta(3) integrins of human mesothelial cells in vitro. Exp Mol Pathol. 1999;66(2):131–139.
Witz CA, Cho S, Montoya-Rodriguez IA, Schenken RS. The alpha(2)beta(1) and alpha(3)beta(1) integrins do not mediate attachment of endometrial cells to peritoneal mesothelium. Fertil Steril. 2002;78(4):796–803.
Witz CA, Montoya-Rodriguez IA, Cho S, Centonze VE, Bonewald LE, Schenken RS. Composition of the extraceullar matrix of the peritoneum. J Soc Gynecol Invest. 2001;8(5): 299–304.
Oral E, Olive DL, Arici A. The peritoneal environment in endometriosis. Hum Reprod Update. 1996;2(5):385–398.
Zhang RJ, Wild RA, Ojago JM. Effect of tumor necrosis factor-alpha on adhesion of human endometrial stromal cells to peritoneal mesothelial cells: an in vitro system. Fertil Steril. 1993;59(6):1196–1201.
Debrock S, De Strooper B, Vander Perre S, Hill JA, D’Hooghe TM. Tumour necrosis factor-alpha, interleukin-6 and interleukin-8 do not promote adhesion of human endometrial epithelial cells to mesothelial cells in a quantitative in vitro model. Hum Reprod. 2006;21(3):605–609.
Sillem M, Prifti S, Monga B, Arslic T, Runnebaum B. Integrin-mediated adhesion of uterine endometrial cells from endometriosis patients to extracellular matrix proteins is enhanced by tumor necrosis factor alpha (TNF alpha) and interleukin-1 (IL-1). Eur J Obstet Gynecol Reprod Biol. 1999; 87(2):123–127.
Iwabe T, Harada T, Tsudo T, et al. Tumor necrosis factor-alpha promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J Clin Endocrinol Metab. 2000;85(2):824–829.
Braun DP, Ding J, Dmowski WP. Peritoneal fluid-mediated enhancement of eutopic and ectopic endometrial cell proliferation is dependent on tumor necrosis factor-alpha in women with endometriosis. Fertil Steril. 2002;78(4):727–732.
Bouhadir KH, Mooney DJ. In vitro and in vivo models for the reconstruction of intercellular signaling. Ann N Y Acad Sci. 1998;842:188–194.
O’Brien LE, Zegers MM, Mostov KE. Opinion: building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3(7):531–537.
Santini MT, Rainaldi G, Indovina PL. Apoptosis, cell adhesion and the extracellular matrix in the three-dimensional growth of multicellular tumor spheroids. Crit Rev Oncol Hematol. 2000;36(2–3):75–87.
Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21(24):6188–6193.
Kleinman HK, McGarvey ML, Hassell JR, et al. Basement membrane complexes with biological activity. Biochemistry. 1986;25(2):312–318.
Kobayashi H. Invasive capacity of heterotopic endometrium. Gynecol Obstet Invest. 2000;50(suppl 1):26–32.
Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol. 2001;159(5):1839–1852.
Nair AS, Nair HB, Lucidi RS, et al. Modeling the early endometriotic lesion: mesothelium-endometrial cell co-culture increases endometrial invasion and alters mesothelial and endometrial gene transcription. Fertil Steril. 2008;90(4 suppl):1487–1495.
Fasciani A, Bocci G, Xu J, et al. Three-dimensional in vitro culture of endometrial explants mimics the early stages of endometriosis. Fertil Steril. 2003;80(5):1137–1143.
Esfandiari N, Khazaei M, Ai J, et al. Effect of a statin on an in vitro model of endometriosis. Fertil Steril. 2007;87(2): 257–262.
Bittinger F, Brochhausen C, Skarke C, Kohler H, Kirkpatrick CJ. Reconstruction of peritoneal-like structure in three-dimensional collagen gel matrix culture. Exp Cell Res. 1997;236(1):155–160.
Yang H, Han S, Kim H, et al. Expression of integrins, cyclooxygenases and matrix metalloproteinases in three-dimensional human endometrial cell culture system. Exp Mol Med. 2002;34(1):75–82.
Griffith JS, Binkley PA, Kirma NB, Schenken RS, Tekmal RR, Witz CA. Imatinib decreases endometrial stromal cell transmesothelial migration and proliferation in the extracellular matrix of modeled peritoneum. Fertil Steril. 2007;88(suppl 1):S60–S61.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Griffith, J.S., Rodgers, A.K. & Schenken, R.S. In Vitro Models to Study the Pathogenesis of Endometriosis. Reprod. Sci. 17, 5–12 (2010). https://doi.org/10.1177/1933719109338221
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719109338221