Abstract
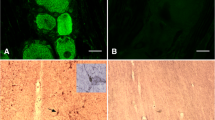
Dynorphin, an endogenous opioid peptide, mediates progesterone-negative feedback on gonadotropin-releasing hormone (GnRH) neurons in other species. The role of dynorphin in humans is unclear. The objective of this study was to determine if dynorphin fibers have close contacts with GnRH neurons in humans. Dual-label immunocytochemistry was peformed on postmortem human hypothalamic tissue. The majority of GnRH neurons, 87.5%, had close contacts with dynorphin fibers and multiple close contacts were common, 62.5%. There were no regional differences between the hypothalamus and preoptic area in the distribution of close contacts. More close contacts were identified on the GnRH dendrites compared to the cell bodies (P < .001), but this difference was not significant when corrected for length. In conclusion, dynorphin fibers form close contacts with GnRH neurons in humans. This neuroanatomical evidence may suggest that dynorphin has effects on GnRH regulation in humans as seen in other species.
Similar content being viewed by others
References
Fritz MA, Speroff L. The endocrinology of the menstrual cycle: the interactions of folliculogenesis and neuroendocrine mechanisms. Fertil Steril. 1982;38(5):509–529.
Brann DW, Mahesh VB. Regulation of gonadotropin secretion by steroid hormones. Front Neuroendocrinol. 1991;12(2):165–207.
Skinner DC, Evans NP, Delaleu B, Goodman RL, Bouchard P, Caraty A. The negative feedback actions of progesterone on gonadotropin-releasing hormone secretion are transduced by the classical progesterone receptor. Proc Natl Acad Sci. 1998;95(18):10978–10983.
Skinner DC, Caraty A, Allingham R. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology. 2001;142(2):573–579.
Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine pre-optic area and hypothalamus. Endocrinology. 2002;143(11):4366–4374.
Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967.
Ferin M. Endogenous opioid peptides and the menstrual cycle. Trends Neurosci. 1984;7(6):194–196.
Moore MR, Black PM. Neuropeptides. Neurosurg Rev. 1991;14(2):97–110.
Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835–1842.
Tan-No K, Takahashi H, Nakagawasai O, et al. Pronociceptive role of dynorphins in uninjured animals:N-ethylmaleimide-induced nociceptive behavior mediated through inhibition of dynorphin degradation. Pain. 2005;113(3):301–309.
Dudas B, Merchenthaler I. Close anatomical associations between β-endorphin and luteinizing hormone-releasing hormone neuronal systems in the human diencephalon. Neuroscience. 2004;124(1):221–229.
Abe J, Okamura H, Kitamura T, et al. Immunocytochemical demonstration of dynorphin (PH–8P)-like immunoreactive elements in the human hypothalamus. J Comp Neurol. 1988;276(4):508–513.
Dudas B, Merchenthaler I. Topography and Associations of Leu-Enkephalin and luteinizing hormone-releasing hormone neuronal systems in the human diencephalon. J Clin Endocrinol Metab. 2003;88(4):1842–1848.
Watson RE Jr, Hoffmann GE, Wiegand SJ. Sexually dimorphic opioid distribution in the preoptic area: manipulation by gonadal steroids. Brain Res. 1986;398(1):157–163.
Simerly RB, McCall LD, Watson SJ. Distribution of opioid peptides in the preoptic region: immunohistochemical evidence for a steroid-sensitive enkephalin sexual dimorphism. J Comp Neurol. 1988;276(3):442–459.
Steele PA, Judd SJ. Role of endogenous opioids in reducing the frequency of pulsatile luteinizing hormone secretion induced by progesterone in normal women. Clin Endocrin. 1986;25(6):669–674.
Ropert JF, Quigley ME, Yen SSC. Endogenous opiates modulate pulsatile luteinizing hormone release in humans. J Clin Endocrinol Metab. 1981;52(3):583–585.
Quigley ME, Yen SSC. Role of endogenous opiates on LH secretion during the menstrual cycle. J Clin Endocrinol Metabol. 1980;51(1):179–181.
Casper RF, Alapin-Rubillovitz S. Progestins increase endogenous opioid peptide activity in postmenopausal women. J Clin Endocrinol Metabol. 1985;60(1):34–36.
Shoupe D, Montz FJ, Lobo RA. The effects of estrogen and progestin on endogenous opioid activity in oophorectomized women. J Clin Endocrinol Metab. 1985;60(1):178–183.
Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215(4531):413–415.
Kalra SP, Kalra PS. Opioid-adrenergic-steroid connection in regulation of luteinizing hormone secretion in the rat. Neuroendocrinology. 1984;38(5):418–426.
Aleem FA, McIntash T. Elevated plasma levels of β-endorphin in a group of women with polycystic ovarian disease. Fertil Steril. 1984;42(5):686–689.
Wildt L, Leyendecker G, Sir-Petermann T, Waibel-Treber S. Treatment with naltrexone in hypothalamic ovarian failure: induction of ovulation and pregnancy. Hum Reprod. 1993;8(3):350–358.
Whisnant CS, Havern RL, Goodman RL. Endogenous opioid suppression of luteinizing hormone pulse frequency and amplitude in the ewe: hypothalamic sites of action. Neuroendocrinology. 1991;54(6):587–593.
Lung FD, Chen CH, Liu JH. Development of highly potent and selective dynorphin a analogues as new medicines. J Pept Res. 2005;66(5):263–276.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dahl, S.K., Amstalden, M., Coolen, L. et al. Dynorphin Immunoreactive Fibers Contact GnRH Neurons in the Human Hypothalamus. Reprod. Sci. 16, 781–787 (2009). https://doi.org/10.1177/1933719109336619
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719109336619