Abstract
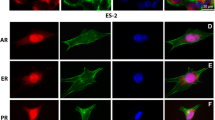
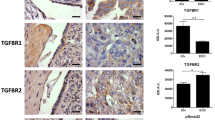
Objectives: An epithelial ovarian cancer cell line constitutively expressing the androgen receptor was created to evaluate the mechanism and effects of androgen receptor activation on epithelial ovarian cancer cell invasion. Methods: Immunocytochemistry and Western blot analyses confirmed androgen receptor expression. Boyden chamber invasion assays were performed using cells treated with the androgen receptor ligands medroxyprogesterone acetate or dihydrotestosterone. The matrix metalloproteinases associated with invasion were investigated using zymographic assays. Results: Androgen receptor—mediated invasion is ligand dependent. While both medroxyprogesterone acetate and dihydrotestosterone signal through androgen receptor, medroxyprogesterone acetate is more effective at stimulating invasion of epithelial ovarian cancer cells. Unlike the wild-type epithelial ovarian cancer cells, this increase in invasion in androgen receptor+ epithelial ovarian cancer cells does not seem to be dependent on matrix metalloproteinase 2 or 9 activation. Conclusion: Although classified as a progestin, medroxyprogesterone acetate has significant androgenic activity unique from the pure androgen dihydrotestosterone. Our studies suggest that pharmacologic doses of medroxyprogesterone acetate may actually increase the invasive potential of epithelial ovarian cancer cells.
Similar content being viewed by others
References
The reduction in risk of ovarian cancer associated with oral-contraceptive use. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. N Engl J Med. 1987;316:650–655.
Risch HA Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90:1774–1786.
Kuhnel R., de Graaff J., Rao BR, Stolk JG Androgen receptor predominance in human ovarian carcinoma. J Steroid Biochem. 1987;26:393–397.
Li AJ, Baldwin RL, Karlan BY Short androgen receptor allele length is a poor prognostic factor in epithelial ovarian carcinoma. Clin Cancer Res. 2003;9:3667–3673.
Edmondson R., Monaghan J., Davies B. The human ovarian surface epithelium is an androgen responsive tissue. Br J Cancer. 2002;86:879–885.
Syed V., Ulinski G., Mok S., Yiu G., Ho S. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. 2001;61:6768–6776.
Kemppainen JA, Langley E., Wong CI, Bobseine K., Kelce WR, Wilson EM Distinguishing androgen receptor agonists and antagonists: distinct mechanisms of activation by medroxyprogesterone acetate and dihydrotestosterone. Mol Endocrinol. 1999;13:440–454.
Ghatge RP, Jacobsen BM, Schittone SA, Horwitz KB The progestational and androgenic properties of medroxyprogesterone acetate: gene regulatory overlap with dihydrotestosterone in breast cancer cells. Breast Cancer Res. 2005;7: R1036–R1050.
Lentz SS, Brady MF, Major FJ, Reid GC, Soper JT High-dose megestrol acetate in advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 1996;14:357–361.
Miller AA, Becher R., Schmidt CG Plasma concentrations of medroxyprogesterone acetate and megesterol acetate during long-term follow-up in patients treated for metastatic breast cancer. J Cancer Res Clin Oncol. 1988;114:186–190.
Thigpen JT, Brady MF, Alvarez RD, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736–1744.
Birrell SN, Roder DM, Horsfall DJ, Bentel JM, Tilley WD Medroxyprogesterone acetate therapy in advanced breast cancer: the predictive value of androgen receptor expression. J Clin Oncol. 1995;13:1572–1577.
Recchione C., Venturelli E., Manzari A., Cavalleri A., Martinetti A., Secreto G. Testosterone, dihydrotestosterone and oestradiol levels in postmenopausal breast cancer tissues. J Steroid Biochem Mol Biol. 1995;52:541–546.
Horwitz KB, Pike AW, Gonzalez-Aller C., Fennessey PV Progesterone metabolism in T47Dco human breast cancer cells-II. Intracellular metabolic path of progesterone and synthetic progestins. J Steroid Biochem. 1986;25:911–916.
Fishman DA, Bafetti LM, Stack MS Membrane-type matrix metalloproteinase expression and matrix metalloproteinase-2 activation in primary human ovarian epithelial carcinoma cells. Invasion Metastasis. 1996;16:150–159.
Fishman DA, Liu Y., Ellerbroek SM, Stack MS Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res. 2001;61:3194–3199.
Wang FQ, So J., Reierstad S., Fishman DA Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase. Int J Cancer. 2005;114:19–31.
Liao X., Thrasher J., Pelling J., Holzbeierlein J., Sang Q., Li B. Androgen stimulates matrix metalloproteinase-2 expression in human prostate cancer. Endocrinology. 2003;144:1656–1663.
Simental JA, Sar M., Lane MV, French FS, Wilson EM Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266:510–518.
Richer JK, Lange CA, Manning NG, Owen G., Powell R., Horwitz KB Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem. 1998;273:31317–31326.
Helzlsouer KJ, Alberg AJ, Gordon GB, et al. Serum gonadotropins and steroid hormones and the development of ovarian cancer. Jama. 1995;274:1926–1930.
Kemppainen JA, Lane MV, Sar M., Wilson EM Androgen receptor phosphorylation, turnover, nuclear transport, and transcriptional activation. Specificity for steroids and antihormones. J Biol Chem. 1992;267:968–974.
Thigpen JT, Brady MF, Alvarez RD, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736–1744.
Hamerlynck JV, Maskens AP, Mangioni C., et al. Phase II trial of medroxyprogesterone acetate in advanced ovarian cancer: an EORTC Gynecological Cancer Cooperative Group Study. Gynecol Oncol. 1985;22:313–316.
Tumolo S., Rao BR, van der Burg ME, Guastalla JP, Renard J., Vermorken JB Phase II trial of flutamide in advanced ovarian cancer: an EORTC Gynaecological Cancer Cooperative Group study. Eur J Cancer. 1994;30A:911–914.
Birrell S., Butler L., Harris J., Buchanan G. Disruption of androgen receptor signaling by synthetic progestins may increase risk of developing breast cancer. FASEB. 2007;21:2285–2293.
Sitruk-Ware R. New progestogens: a review of their effects in perimenopausal and postmenopausal women. Drugs Aging. 2004;21:865–883.
Saner KJ, Welter BH, Zhang F., et al. Cloning and expression of a novel, truncated, progesterone receptor. Mol Cell Endocrinol. 2003;200:155–163.
So J., Wang FQ, Navari J., Schreher J., Fishman DA LPAinduced epithelial ovarian cancer (EOC) in vitro invasion and migration are mediated by VEGF receptor-2 (VEGF-R2). Gynecol Oncol. 2005;97:870–878.
Ariztia EV, Lee CJ, Gogoi R., Fishman DA The tumor microenvironment: key to early detection. Crit Rev Clin Lab Sci. 2006;43:393–425.
Rabbani S., Mazar A. The role of the plasminogen activation system in angiogenesis and metastasis. Surg Oncol Clin N Am. 2001;10:393–415.
Di Nezza LA, Jobling T., Salamonsen LA Progestin suppresses matrix metalloproteinase production in endometrial cancer. Gynecol Oncol. 2003;89:325–333.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gogoi, R., Kudla, M., Gil, O. et al. The Activity of Medroxyprogesterone Acetate, an Androgenic Ligand, in Ovarian Cancer Cell Invasion. Reprod. Sci. 15, 846–852 (2008). https://doi.org/10.1177/1933719108323446
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719108323446