Abstract
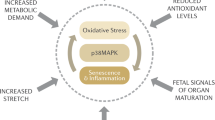
The human fetal membranes are complex tissues that perform many important functions during gestation. The extracellular matrix provides their strength to withstand the forces directed from the fetus and myometrium. Relaxin is a collagenolytic hormone that causes increased production of the matrix metalloproteinases. Its expression from the decidua is increased in patients with preterm premature rupture of the membranes, and its leucine-rich G receptor 7 is upregulated at preterm. The authors previously showed that relaxin is not involved in the infection-mediated cytokine response, but in the absence of infection, it causes increased secretion of both interleukin -6 and interleukin-8 from the membranes. In this article, the authors propose that relaxin is one of a number of sterile stimuli capable of causing increased proinflammatory cytokines, similar to but less robust than the effects of infection. These probably represent distinct inflammatory pathways involving different intracellular signaling events, which can result in either preterm premature rupture of the membranes or preterm labor. The current challenge is to fully understand these pathways and to clarify their similarities and differences.
Similar content being viewed by others
References
Bryant-Greenwood GD The extracellular matrix of the human fetal membranes: structure and function. Placenta. 1998;19:1–11.
Pressman EK, Cavanaugh JL, Woods JR Physical properties of the chorioamnion throughout gestation. Am J Obstet Gynecol. 2002;187:672–675.
Parry S., Strauss JF Premature rupture of the fetal membranes. N Engl J Med. 1998;338:663–670.
Koay ESC, Too CKL, Greenwood FC, et al. Relaxin stimulates collagenase and plasminogen activator secretion by dispersed human amnion and chorion cells in vitro. J Clin Endocr Metab. 1983;56:1332–1334.
Tashima LS, Yamamoto SY, Yasuda M, et al. Decidual relaxins: gene and protein upregulation in preterm premature rupture of the membranes by complementary DNA arrays and quantitative immunocytochemistry. Am J Obstet Gynecol. 2002;187:785–797.
Qin X., Garibay-Tupas JL, Chua PK, et al. An autocrine/paracrine role of human decidual relaxin. I. Interstitial collagenase (MMP-1) and tissue plasminogen activator. Biol Reprod. 1997;56:800–811.
Qin X., Chua PK, Ohira RH, et al. An autocrine/paracrine role of human decidual relaxin. II. Stromelysin-1 (MMP-3) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1). Biol Reprod. 1997;56:812–820.
Garibay-Tupas JL, Maaskant RA, Greenwood FC, et al. Characteristics of the binding of 32P-labelled human relaxins to the human fetal membranes. J Endocr. 1995;145:441–448.
Lowndes K., Amano A., Yamamoto SY, et al.The human relaxin receptor (LGR7): expression in the fetal membranes and placenta. Placenta. 2006;27:610–618.
Mazella J., Tang M., Tseng L. Disparate effects of relaxin and TGFβ1: relaxin increases, but TGFβ1 inhibits, the relaxin and relaxin receptor and production of IGFBP-1 in human endometrial stromal/decidual cells. Hum Reprod. 2004;19: 1513–1518.
Kern A., Agoulnik AI, Bryant-Greenwood GD The LDL-A module of the relaxin receptor (LGR7): its role in signaling and trafficking to the cell membrane. Endocrinology. 2007;148: 1181–1194.
Kern A., Amano A., Bryant-Greenwood GD Identification and functional characterization of the relaxin receptor (LGR7) splice variants in the human decidua. Presented at: the Society for Gynecologic Investigation; 2007. Abstract 322.
Oxlund H., Helmig R., Halaburt JT, et al. Biomechanical analysis of human chorioamniotic membranes. Eur J Obstet Gynecol Reprod Biol. 1990;34:247–255.
Benson-Martin J., Zammaretti P., Bilic G, et al. The Young’s modulus of fetal preterm and term amniotic membranes. Eur J Obstet Gynecol Reprod Biol. 2006;128:103–107.
Oyen ML, Calvin SE, Landers DV Premature rupture of the fetal membranes: is the amnion the major determinant?Am J Obstet Gynecol. 2006;195:510–515.
Moore RM, Mansour JM, Redline RW, et al.The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2006;27:1037–1051.
Arikat S., Novince RW, Mercer BM, et al. Separation of amnion from choriodecidua is an integral event to the rupture of normal term fetal membranes and constitutes a significant component of the work required. Am J Obstet Gynecol. 2006;194:211–217.
Meinert M., Eriksen GV, Petersen AC, et al. Proteoglycans and hyaluronan in human fetal membranes. Am J Obstet Gynecol. 2001;184:679–685.
Fawthrop RK, Ockleford CD Cryofracture of human term amniochorion. Cell Tissue Res. 1994;277:315–323.
Ockleford C., Malak T., Hubbard A, et al. Confocal and conventional immunofluorescence and ultrastructural localization of intracellular strength-giving components of human amniochorion. J Anat. 1993;183:483–505.
Malak TM, Bell SC Structural characteristics of term human fetal membranes: a novel zone of extreme morphological alteration within the rupture site. Br J Obstet Gynecol. 1994; 101:375–386.
Quintero RA, Morales WJ, Kalter CS, et al. Transabdominal intra-amniotic endoscopic assessment of previable premature rupture of membranes. Am J Obstet Gynecol. 1998;179:71–76.
Khwad ME, Stetzer B., Moore RM, et al. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before the onset of labor. Biol Reprod. 2005;72:720–726.
Osman I., Young A., Jordan F, et al. Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J Soc Gynecol Investig. 2006;13:97–103.
Addad R., Tromp G., Kuivaniemi H, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394–405.
Beutler B. Inferences, questions and possibilities in toll-like receptor signaling. Nature. 2004;430:257–263.
Ozato K., Tsujimura H., Tamura T. Toll-like receptor signaling and regulation of cytokine gene expression in the immune system. Biotechniques. 2002;33:S66–S75.
Sadowsky DW, Adams KM, Gravett MG, et al. Preterm labor is induced by intraamniotic infusions of interleukin-1β and tumor necrosis factor-α but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–1589.
Werner SL, Barken D., Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861.
Hoebe K., Janssen E., Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5:971–974.
Krikun G., Lockwood CJ, Abrahams VM, et al. Expression of toll-like receptors in the human decidua. Histol Histopathol. 2007;22:847–854.
Shim S-S., Romero R., Hong J-S, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191: 1339–1345.
Millar LK, Boesche M., Yamamoto SY, et al. A relaxin-mediated pathway to preterm premature rupture of the fetal membranes that is independent of infection. Am J Obstet Gynecol. 1998;179: 126–134.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from NIH HD 24314, HD 06159, HD 18185, HD 01264, and HD 41676.
Rights and permissions
About this article
Cite this article
Bryant-Greenwood, G.D., Kern, A., Yamamoto, S.Y. et al. Relaxin and the Human Fetal Membranes. Reprod. Sci. 14 (Suppl 8), 42–45 (2007). https://doi.org/10.1177/1933719107310821
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719107310821