Abstract
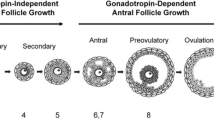
Regulation of ovarian follicle development depends on endocrine- and paracrine-acting hormones, the 3-dimensional architecture of the follicle, and the physical rigidity of the surrounding tissue. These 3 forces are integrated throughout the life cycle of the follicle to ensure appropriate hormone secretion, differentiation of the somatic cells, and maturation of the oocyte. The process of in-follicle maturation provides a new tool for understanding ovarian follicle development under the influence of these factors.
Similar content being viewed by others
References
Zhao Y., Luck MR Gene expression and protein distribution of collagen, fibronectin and laminin in bovine follicles and corpora lutea. J Reprod Fertil. 1995;104:115–123.
Rodgers HF, Irvine CM, van Wezel IL, et al. Distribution of the α1 to α6 chains of type IV collagen in bovine follicles. Biol Reprod. 1998;59:1334–1341.
McArthur ME, Irving-Rodgers HF, Byers S., Rodgers RJ Identification and immunolocalization of decorin, versican, perlecan, nidogen, and chondroitin sulfate proteoglycans in bovine small-antral ovarian follicles. Biol Reprod. 2000;63: 913–924.
Rodgers RJ, Irving-Rodgers HF, van Wezel IL, Krupa M., Lavranos TC Dynamics of the membrana granulosa during expansion of the ovarian follicular antrum. Mol Cell Endocrinol. 2001; 171:41–48.
Yamada S., Fujiwara H., Honda T, et al. Human granulosa cells express integrin α2 and collagen type IV: possible involvement of collagen type IV in granulosa cell luteinization. Mol Hum Reprod. 1999;5:607–617.
Iwahashi M., Muragaki Y, Ooshima A, Nakano R. Type VI collagen expression during growth of human ovarian follicles. Fertil Steril. 2000; 74:343–347.
Rajah R., Sundaram GS Protein distribution and gene expression of collagen type IV in the neonatal rat ovary during follicle formation. Cell Mol Biol (Noisy-le-grand). 1994; 40:769–780.
Frojdman K., Pelliniemi LJ, Virtanen I. Differential distribution of type IV collagen chains in the developing rat testis and ovary. Differentiation. 1998; 63:125–130.
Huet C., Pisselet C., Mandon-Pepin B., Monget P., Monniaux D. Extracellular matrix regulates ovine granulosa cell survival, proliferation and steroidogenesis: relationships between cell shape and function. J Endocrinol. 2001;169:347–360.
Le Bellego F., Pisselet C., Huet C., Monget P., Monniaux D. Laminin-α6β1 integrin interaction enhances survival and proliferation and modulates steroidogenesis of ovine granulosa cells. J Endocrinol. 2002; 172:45–59.
Gentry PA, Zareie M., Liptrap RM Fibronectin concentrations correlate with ovarian follicular size and estradiol values in equine follicular fluid. Anim Reprod Sci. 1996;45:91–102.
Berkholtz et al. In press.
Ben-Ze’ev A., Amsterdam A. Regulation of cytoskeletal proteins involved in cell contact formation during differentiation of granulosa cells on extracellular matrix. Proc Natl Acad Sci U S A. 1986;83:2894–2898.
Ben-Rafael Z., Benadiva CA, Mastroianni L. Jr., et al. Collagen matrix influences the morphologic features and steroid secretion of human granulosa cells. Am J Obstet Gynecol. 1988; 159: 1570–1574.
Furman A., Rotmensch S., Dor J, et al. Culture of human granulosa cells from an in vitro fertilization program: effects of extracellular matrix on morphology and cyclic adenosine 30,50 monophosphate production. Fertil Steril. 1986;46:514–517.
Asem EK, Feng S., Stingley-Salazar SR, et al. Basal lamina of avian ovarian follicle: influence on morphology of granulosa cells in-vitro. Comp Biochem Physiol Part C. 2000;125:189–201.
Bussenot I., Ferre G., Azoulay-Barjonet C, et al. Culture of human preovulatory granulosa cells: effect of extracellular matrix on steroidogenesis. Biol Cell. 1993;77:181–186.
Carnegie JA, Byard R., Dardick I., Tsang BK Culture of granulosa cells in collagen gels: the influence of cell shape on steroidogenesis. Biol Reprod. 1988;38:881–890.
Gomes JE, Correia SC, Gouveia-Oliveira A., Cidadao AJ, Plancha CE Three-dimensional environments preserve extracellular matrix compartments of ovarian follicles and increase FSH-dependent growth. Mol Reprod Dev. 1999; 54:163–172.
Hwang DH, Kee SH, Kim K, et al. Role of reconstituted basement membrane in human granulosa cell culture. Endocr J. 2000;47:177–183.
Maresh GA, Timmons TM, Dunbar BS Effects of extracellular matrix on the expression of specific ovarian proteins. Biol Reprod. 1990;43:965–976.
Amsterdam A., Rotmensch S., Furman A., Venter EA, Vlodavsky I. Synergistic effect of human chorionic gonadotropin and extracellular matrix on in vitro differentiation of human granulosa cells: progesterone production and gap junction formation. Endocrinology. 1989;124:1956–1964.
Rowley JA, Madlambayan G., Mooney DJ Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53.
Kidder GM, Mhawi AA Gap junctions and ovarian folliculogenesis. Reproduction. 2002;123:613–620.
Richardson MC, Slack C., Stewart IJ Rearrangement of extracellular matrix during cluster formation by human luteinising granulosa cells in culture. J Anat. 2000;196(pt 2): 243–248.
Aharoni D., Meiri I., Atzmon R., Vlodavsky I., Amsterdam A. Differential effect of components of the extracellular matrix on differentiation and apoptosis. Curr Biol. 1997;7:43–51.
Ruoslahti E., Reed JC Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478.
Adams JC, Watt FM Regulation of development and differentiation by the extracellular matrix. Development. 1993;117: 1183–1198.
Bortolussi M., Zanchetta R., Doliana R, et al. Changes in the organization of the extracellular matrix in ovarian follicles during the preovulatory phase and atresia: an immunofluorescence study. BasicAppl Histochem. 1989;33:31–38.
Irving-Rodgers HF, Mussard ML, Kinder JE, Rodgers RJ Composition and morphology of the follicular basal lamina during atresia of bovine antral follicles. Reproduction. 2002;123:97–106.
Yasuda K., Hagiwara E., Takeuchi A, et al. Changes in the distribution of tenascin and fibronectin in the mouse ovary during folliculogenesis, atresia, corpus luteum formation and luteolysis. ZoologSci. 2005;22:237–245.
Hovatta O., Silye R., Abir R., Krausz T., Winston RM Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032–1036.
Gospodarowicz D., Delgado D., Vlodavsky I. Permissive effect of the extracellular matrix on cell proliferation in vitro. Proc Natl Acad Sci U S A. 1980;77:4094–4098.
Kreeger PK, Deck JW, Woodruff TK, Shea LD The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–723.
Oktay K., Karlikaya G., Akman O., Ojakian GK, Oktay M. Interaction of extracellular matrix and activin-A in the initiation of follicle growth in the mouse ovary. Biol Reprod. 2000; 63:457–461.
Kreeger PK, Woodruff TK, Shea LD Murine granulosa cell morphology and function are regulated by a synthetic Arg-Gly-Asp matrix. Mol Cell Endocrinol. 2003; 205:1–10.
Furman A., Rotmensch S., Kohen F., Mashiach S., Amsterdam A. Regulation of rat granulosa cell differentiation by extracellular matrix produced by bovine corneal endothelial cells. Endocrinology. 1986;118:1878–1885.
Aten RF, Kolodecik TR, Behrman HR A cell adhesion receptor antiserum abolishes, whereas laminin and fibronectin glycoprotein components of extracellular matrix promote luteinization of cultured rat granulosa cells. Endocrinology. 1995;136:1753–1758.
Asem EK, Stingley-Salazar SR, Robinson JP, Turek JJ Effect of basal lamina on progesterone production by chicken granulosa cells in vitro—influence of follicular development. Comp Biochem Physiol Part C. 2000; 125:233–244.
Sites CK, Kessel B., LaBarbera AR Adhesion proteins increase cellular attachment, follicle-stimulating hormone receptors, and progesterone production in cultured porcine granulosa cells. Proc Soc Exp Biol Med. 1996;212:78–83.
Wang X., Otsu K., Saito H., Hiroi M., Ishikawa K. Sandwich configuration of type I collagen suppresses progesterone production in primary cultured porcine granulosa cells by reducing gene expression of cytochrome P450 cholesterol side-chain cleavage enzyme. Arch Biochem Biophys. 2000;376:117–123.
Emmen JM, Couse JF, Elmore SA, et al. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER)α and ERβ null mice indicate a role for ERβ in follicular maturation. Endocrinology. 2005;146:2817–2826.
Rodgers RJ, Vella CA, Rodgers HF, Scott K., Lavranos TC Production of extracellular matrix, fibronectin and steroidogenic enzymes, and growth of bovine granulosa cells in anchorage independent culture. Reprod Fertil Dev. 1996;8:249–257.
O’Shea JD Heterogeneous cell types in the corpus luteum of sheep, goats and cattle. J Reprod Fertil Suppl. 1987;34:71–85.
Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005; 73:942–950.
Pangas SA A novel in vitro culture system for the analysis of follicle development. Tissue Eng. 2003;9:1013–1021.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woodruff, T.K., Shea, L.D. The Role of the Extracellular Matrix in Ovarian Follicle Development. Reprod. Sci. 14 (Suppl 8), 6–10 (2007). https://doi.org/10.1177/1933719107309818
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719107309818