Abstract
Objective
TO test the hypothesis that interleukin-1β (IL- 1β) and tumor necrosis factor-α (TNF-α) regulate granulocyte colony-stimulating factor (G-CSF) production by human placental villous core mesenchymal cells.
Methods
Villous core mesenchymal cells were isolated from placentas at 14—20 weeks’ gestation and cultured in vitro. Cells were treated with IL-1β or TNF-α in dose-response and time-course studies. We measured G-CSF mRNA expression by Northern blot analysis and G-CSF protein production by enzyme-linked immunosorbent assay of the conditioned media.
Results
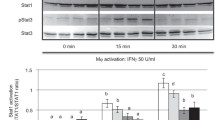
Unstimulated mesenchymal cells expressed negligible G-CSF. Steady-state G-CSF mRNA expression was maximal 3–6 hours after IL- 1β treatment and 6–18 hours after TNF-α treatment. Each cytokine induced G-CSF protein production in dose- and time-dependent manners, with IL-1β more potent than TNF-α. The G-CSF mRNA expression and G-CSF protein production induced by the combination of both cytokines exceeded that induced by either cytokine alone.
Conclusions
Interleukin-1β and TNF-α stimulate G-CSF production by placental villous core mesenchymal cells in vitro. These results identify a potential mechanism by which villous core mesenchymal cells mediate, in part, the placental response to these two cytokines. (J Soc Gynecol Invest 1996;3:172-8)
Similar content being viewed by others
References
Le J, Vilcek J. Tumor necrosis factor and interleukin-1: Cytokines with multiple overlapping biological activities. Lab Invest 1987;56:234.
Simon C, Frances A, Piquette GN, Danasouri IE, Zurawski G, Dang W, Polan ML. Embryonic implantation in mice is blocked by interleukin-1 receptor antagonist. Endocrinology 1994;134:521–8.
Tartakowsky B, Ben-Yair E. Cytokines modulate pre-implantation development and pregnancy. Dev Biol 1991;146:345–52.
Vince G, Shorter S, Starkey P, et al. Localization of tumor necrosis factor production in cells at the matemo/fetal interface in human pregnancy. Clin Exp Immunol 1992;88:174–80.
Kauma SW, Matt D, Strom S, Eierman D, Turner T. Interleukin-1β, human leukocyte antigen HLA-DR alpha and transforming growth factor-β expression in endometrium, placenta, and placental membranes. Am J Obstet Gynecol 1990;163:1430–7.
Wegman TG, Athanassakis I, Guilbert L, et al. The role of M-CSF and GM-CSF in fostering placental growth, fetal growth, and fetal survival. Transplant Proc 1989;21:566–8.
Yagel S, Peeyush K, Powell W, Casper R. Interleukm-1 stimulates human chorionic gonadotropin secretion by first tnmester human trophoblast. J Clin Endocrinol Metab 1989;68:9992–5.
Nestler JE. Interleukin-1 stimulates the aromatase activity of human placental cytotrophoblasts. Endocrinology 1993;132:566–70.
Ohashi K, Saji F, Kato M, Wakimoto A, Tamzawa O. Tumor necrosis factor-α inhibits human chorionic gonadotropin secretion. J Clin Endocrinol Metab 1992;74:130–4.
Clark SC, Kamen R. The human hematopoietic colony-stimulating factors. Science 1987;236:1229–37.
Queensberry PJ, McNiece IK, McGrath HE, Temeles DS, Baber GB, Deacon DH. Stromal cell regulation of hematopoiesis. Ann N Y Acad Sci 1989;554:116–24.
Caldwell J, Emerson SG. IL-1α and TNFα act synergistically to stimulate production of myeloid colony-stimulating factors by cultured human bone marrow stromal cells and cloned stromal cell strains. J Cell Physiol 1994;159:221–8.
Kauma SW, Walsh SW, Nestler JE, Turner T. Interleukin-1 is induced in the human placenta by endotoxin and isolation procedures for trophoblasts. J Clin Endocrinol Metab 1992;75:951–5.
Harty JH, Kauma SW. Interleukin-1β stimulates colony-stimulating factor-1 production in placental villous core mesenchymal cells. J Clin Endocrinol Metab 1992;75:947–50.
Kauma SW, Turner TT, Harty JR. Interleukin-1β stimulates interleukin-6 production in placental villous core mesenchymal cells. Endocrinology 1994;134:457–60.
Garcia-Lloret MI, Morrish DW, Wegman TG, Honore L, Turner AR, Guilbert LJ. Demonstration of functional cytokineplacental interactions: CSF-1 and GM-CSF stimulate human cytotrophoblast differentiation and peptide hormone secretion. Exp Cell Res 1994;214:46–54.
Nishino E, Matsuzaki N, Masuhiro K, et al. Trophoblast-derived interleukin-6 (IL-6) regulates human chorionic gonadotropin release through IL-6 receptor on human trophoblasts. J Clin Endocrinol Metab 1990;71:436–41.
Shorter SC, Vince GS, Starkey PM. Production of granulocyte-colony stimulating factor at the materno-fetal interface in human pregnancy. Immunology 1992;75:468–74.
Berdel W, Danhauser-Riedl S, Steinhauser G, Winton E. Various human hematopoietic growth factors (interleukin-3, GM-CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood 1989;73:80–3.
Fukunaga R, Seto Yoshiyuki, Mizushima S, Nagata S. Three different mRNAs encoding human granulocyte colony-stimulating factor receptor. Proc Natl Acad Sci U S A 1990;87:8702–6.
Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987;162:156–9.
Tweardy DJ, Cannizzaro LA, Palumbo AP, et al. Molecular cloning and characterization of a cDNA for human granulocyte colony-stimulating factor (G-CSF) from a glioblastoma multiforme cell line and localization of the G-CSF gene to chromosome band 17q21. Oncogene Res 1987;1:209–20.
Uzumaki H, Okabe T, Sasaki N, et al. Identification and characterization of receptors for granulocyte colony-stimulating factor on human placenta and trophoblastic cells. Proc Natl Acad Sci U S A 1989;86:9323–6.
Bussolino F, Wang JM, Defilippi P, et al. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature 1989;337:471–3.
Alenghat E, Esterly JR. Intravascular hematopoiesis in chorionic villi. Am J Clin Pathol 1983;79:225–7.
Starnes HF. Biological effects and possible clinical applications of interleukin-1. Semin Hematol 1991;28:34–41.
Sherry B, Cerami A. Cachectin/tumor necrosis factor exerts endocrine, paracrine, and autocrine control of inflammatory responses. J Cell Biol 1988;107:1269–77.
Gessler P, Kirchmann N, Kientsch-Engel R, Haas N, Lasch P, Kachel W. Serum concentrations of granulocyte colony-stimulating factor in healthy term and preterm neonates and in those with various diseases including bacterial infections. Blood 1993;82:3177–82.
Author information
Authors and Affiliations
Additional information
This work was supported in parr by A. D. Williams Fund Grant 6-6746 (DTV) and National Institutes of Health grant HD29023 (SWK).
Rights and permissions
About this article
Cite this article
Vandermolen, D.T., Kauma, S.W. & Turner, T.T. Interleukin-1β and Tumor Necrosis Factor-α Stimulate Granulocyte Colony-Stimulating Factor Production by Placental Villous Core Mesenchymal Cells. Reprod. Sci. 3, 172–178 (1996). https://doi.org/10.1177/107155769600300403
Published:
Issue Date:
DOI: https://doi.org/10.1177/107155769600300403