Abstract
Objectives
Polypeptide growth factors may modulate the actions of estrogen (E2) and progesterone (P) in reproductive tissues in an autocrine/paracrine manner. The objective of this study was to determine whether the baboon oviduct contains epidermal growth factor (EGF), transforming growth factor-α (TGFα), and EGF receptor (EGF-R) and whether changes in their expression are correlated with various hormonal states.
Methods
Oviductal tissue was obtained from adult female baboons (Tapio anubis) after oophorectomy and steroid treatment, and during the menstrual cycle. Ampullary regions were fixed in Bouin’s fixative and embedded in paraffin for immunocytochemistry using rabbit polyclonal antibodies against EGF and EGF-R, and mouse monoclonal antibody against TGFa.
Results
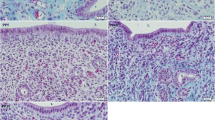
Both EGF and EGF-R were present in all tissue compartments (most strongly in the epithelium, followed by smooth muscle and stroma) at all reproductive stages and showed similar staining patterns. However, the most intense immunoreactive product was found in the tissue obtained from the E2-treated and late follicular phase animals. At this time, intense staining was present in the apical regions of the mature ciliated cells, whereas the stain was dispersed uniformly over the cytoplasm of all other cell types. Immunoreactive TGFa. was limited primarily to the nonciliated epithelial cells, and staining was most intense in the E2-treated and late follicular phase tissues. Transforming growth factor-a formed intense perinuclear deposits in the mature secretory cells, an area that corresponds to the Golgi region. No immunoreactive product was observed for any of these proteins when preimmune serum was substituted for the primary antibody or when the primary antibody was preabsorbed with antigen.
Conclusion
In summary, EGF, TGFa, and EGF-R are present in the ampulla of the baboon oviduct. Moreover, the localization and intensity of immunoreactive product are dependent on cell type and hormonal state. These data are consistent with the concept that EGF, TGFa, and EGF-R may be regulated by E2 and P and thus may play a role in cell differentiation and function. In addition, the specific localization of TGFa suggests that this growth factor may be synthesized for release from the secretory cells and thus may also function as a modulator of gamete/embryo viability and development.
Similar content being viewed by others
References
Verhage HG, Jaffe RC. Hormonal control of the mammalian oviduct: Morphological features and the steroid receptor systems. In: Siegler AM, Ansari AH, eds. The fallopian tube: Basic studies and clinical contributions. New York: Futura Publishing, 1986: 107–18.
Brenner RM, Maslar IA. The primate oviduct and endometrium. In: Knobil E, Neil J, eds. The physiology of reproduction. New York: Raven Press, 1988;303–29.
Verhage HG, Jaffe RC, Fazleabas AT. Steroid-dependent oviduct secretions in the primate. Arch Biol Med Exp 1991;24: 301–9.
Brenner RM. Renewal of oviduct cilia during the menstrual cycle of the rhesus monkey. Fertil Steril 1969;20: 599–611.
Odor DL, Gaddum-Rosse P, Rumery RE, Blandau RJ. Cyclic variations in the oviductal ciliated cells during the menstrual cycle and after estrogen treatment in the pig-tailed monkey, Macaca nemestrina. Anat Rec 1980;198: 35–7.
Brenner RM, Carlisle KS, Hess DL, Sandow BA, West NB. Morphology of the oviduct and endometria of cyno-molgus macaques during the menstrual cycle. Biol Reprod 1983;29: 1289–302.
Odor DL, Gaddum-Rosse P, Rumery RE. Secretory cells of the oviduct of the pig-tailed monkey, Macaca nemestrina, during the menstrual cycle and after estrogen treatment. Am J Anat 1983;166: 149–72.
Verhage HG, Mavrogianis PA, Boice ML, Li W, Fazleabas AT. Oviductal epithelium of the baboon: Hormonal control and immunogold localization of oviduct-specific glycoproteins. Am J Anat 1990;187: 81–90.
Mukku VR, Stancel GM. Regulation of epidermal growth factor receptor by estrogen. J Biol Chem 1985;260: 9820–4.
Mukku VR, Stancel GM. Receptors for epidermal growth factor in the rat uterus. Endocrinology 1985;117: 149–54.
Dickson RB, Lippman ME. Estrogenic regulation of growth and polypeptide growth factor secretion in human breast carcinoma. Endocr Rev 1987;8: 29–43.
Murphy LJ, Murphy LC, Friesen HG. Estrogen induces insulin-like growth factor-1 expression in the rat uterus. Mol Endocrinol 1987;1: 445–50.
DiAugustine RP, Petruez P, Bell GI, et al. Influences of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology 1988;122: 2355–63.
Huet-Hudson YM, Chakraborty C, De SK, Suzuki Y, Andrews GK, Dey SK. Estrogen regulates synthesis of epidermal growth factor in mouse uterine epithelial cells. Mol Endocrinol 1990;4: 510–23.
Nelson KG, Takahashi T, Bossert NL, Walmer DK, McLachlan JA. Epidermal growth factor replaces estrogen in the stimulation of female genital tract growth and differentiation. Proc Natl Acad Sci USA 1991;88: 21–5.
Ignar-Trowbridge DM, Teng CT, Ross KA, Parker MG, Korach KS, McLachlan JA. Peptide growth factors elicit estrogen receptor-dependent transcriptional activation of an estrogen-responsive element. Mol Endocrinol 1993;7: 992–8.
Nelson KG, Takahashi T, Lee DC, et al. Transforming growth factor-a is a potential mediator of estrogen action in the mouse uterus. Endocrinology 1992;131: 1654–64.
Burgess WW. Epidermal growth factor and transforming growth factors. Br Med Bull 1989;45: 101–24.
Lei ZM, Rao ChV. Expression of epidermal growth factor (EGF) receptor and its ligands, EGF and transforming growth factor-a, in human fallopian tubes. Endocrinology 1992;131: 947–57.
Morishige K-I, Kurachi H, Amemiya K, et al. Menstrual stage-specific expression of epidermal growth-factor and transforming growth factor-a in human oviduct epithelium and their role in early embryogenesis. Endocrinology 1993;133: 199–207.
Verhage HG, Boice ML, Mavrogianis P, Donnelly K, Fazleabas AT. Immunological characterization and immuno-cytochemical localization of oviduct-specific glycoproteins in the baboon (Papio anubis). Endocrinology 1989;124: 2464–72.
Verhage HG, Fazleabas AT, Donnelly KD. The in-vitro synthesis and release of proteins by the human oviduct. Endocrinology 1988;122: 1639–45.
Paria BC, Dey SK. Preimplantation embryo development in vitro: Cooperative interactions among embryos and role of growth factors. Proc Natl Acad Sci USA 1990;87: 4756–60.
Rappolee DA, Brenner CA, Schultz R, Mark D, Werb Z. Developmental expression of PDGF, TGF-a and TGF-β genes in preimplantation mouse embryos. Science 1988;241: 1823–5.
Thibodeaux JK, Del Vecchio RP, Hansel W. Role of platelet-derived growth factor in development of in vitro matured and in vitro fertilized bovine embryos. J Reprod Fertil 1993;98: 61–6.
Fazleabas AT, Miller JB, Verhage HG. Synthesis and release of estrogen and progesterone dependent proteins by the baboon (Papio anubis) uterine endometrium. Biol Reprod 1988;39: 729–36.
Carlsson B, Hillension T, Nilsson A, Torneil J, Billig H. Expression of insulin-like growth factor-I (IGFBP-I) in the rat fallopian tube: Possible autocrine and paracrine action of fallopian tube-derived IGF-I on the fallopian tube and on the preimplantation embryo. Endocrinology 1993;133: 2031–9.
Rapisarda JJ, Mavrogianis PA, O’Day-Bowman MB, Fazleabas AT, Verhage HG. Immunological characterization and immunocytochemical localization of an oviduct-specific glycoprotein in the human. J Clin Endocrinol Metab 1993;76: 1483–8.
Bongso A, Ng SC, Fong CY, Ratnam S. Co-cultures: A new lead in embryo quality improvement for assisted reproduction. Fertil Steril 1991;56: 179–89.
Bongso A, Ng S-C, Fong C-Y, et al. Improved pregnancy rate after transfer of embryos grown in human fallopian tubal cell co-culture. Fertil Steril 1992;58: 569–74.
Yeung WSB, Ho PC, Lau EYL, Chan STH. Improved development of human embryos in vitro by a human oviductal cell co-culture system. Hum Reprod 1992;7: 1144–9.
Freeman MR, Bastias MC, Hill GA, Osteen KG. Co-culture of mouse embryos with cells isolated from the human ovarian follicle, oviduct and uterine endometrium. Fertil Steril 1993;59: 138–42.
Author information
Authors and Affiliations
Additional information
Supported by National Institutes of Health grant HD20571.
Rights and permissions
About this article
Cite this article
Schell, D.L., Mavrogianis, P.A., Fazleabas, A.T. et al. Epidermal Growth Factor, Transforming Growth Factor-α, and Epidermal Growth Factor Receptor Localization in the Baboon (Papio anubis) Oviduct During Steroid Treatment and the Menstrual Cycle. Reprod. Sci. 1, 269–276 (1994). https://doi.org/10.1177/107155769400100405
Published:
Issue Date:
DOI: https://doi.org/10.1177/107155769400100405